Preconditioning with partial caloric restriction confers long-term protection against grey and white matter injury after transient focal ischemia
- PMID: 29972653
- PMCID: PMC6668518
- DOI: 10.1177/0271678X18785480
Preconditioning with partial caloric restriction confers long-term protection against grey and white matter injury after transient focal ischemia
Abstract
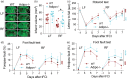
Caloric restriction (CR) has been extensively examined as a preventative strategy against aging and various diseases, but CR effects on cerebral ischemia are largely unknown. We subjected C57BL6/J mice to ad libitum food access (LF) or a diet restricted to 70% of ad libitum food access (RF) for two to four weeks followed by 60 min of transient focal ischemia (tFCI). RF for four weeks protected against subsequent tFCI-induced infarct. RF improved sensorimotor function after stroke in the foot fault and corner tests, as well as performance in the Morris water maze test. In addition, RF preserved ischemic white matter tract integrity assessed by histology and compound action potential. Sirt1 and Sirt3 were both upregulated in RF ischemic brain, but heterozygous deletion of Sirt1 or knockout of Sirt3 did not alter the protection induced by RF against ischemic injury. RF induced significant release of adiponectin, a hormone related to glucose metabolism. Knockout of adiponectin decreased RF-induced protection after tFCI. These data demonstrate the novel finding that white matter, as well as neurons, benefit from CR prior to cerebral ischemic injury, and that adiponectin may contribute to these protective effects.
Keywords: Adiponectin; caloric restriction; cerebral ischemia; sirtuin; stroke.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

