A loss of a velocity-duration trade-off impairs movement precision in patients with cerebellar degeneration
- PMID: 29972715
- PMCID: PMC6175307
- DOI: 10.1111/ejn.14062
A loss of a velocity-duration trade-off impairs movement precision in patients with cerebellar degeneration
Abstract
Current theories discussing the role of the cerebellum have been consistently pointing towards the concept of motor learning. The unavailability of a structure for motor learning able to use information on past errors to change future movements should cause consistent metrical deviations and an inability to correct them; however, it should not boost "motor noise." However, dysmetria, a loss of endpoint precision and an increase in endpoint variability ("motor noise") of goal-directed movements is the central aspect of cerebellar ataxia. Does the prevention of dysmetria or "motor noise" by the healthy cerebellum tell us anything about its normal function? We hypothesize that the healthy cerebellum is able to prevent dysmetria by adjusting movement duration such as to compensate changes in movement velocity. To address this question, we studied fast goal-directed index finger movements in patients with global cerebellar degeneration and in healthy subjects. We demonstrate that healthy subjects are able to maintain endpoint precision despite continuous fluctuations in movement velocity because they are able to adjust the overall movement duration in a fully compensatory manner ("velocity-duration trade-off"). We furthermore provide evidence that this velocity-duration trade-off accommodated by the healthy cerebellum is based on a priori information on the future movement velocity. This ability is lost in cerebellar disease. We suggest that the dysmetria observed in cerebellar patients is a direct consequence of the loss of a cerebellum-based velocity-duration trade-off mechanism that continuously fine-tunes movement durations using information on the expected velocity of the upcoming movement.
Keywords: cerebellar ataxia; duration adjustment; motor noise; precision.
© 2018 The Authors. European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Figures
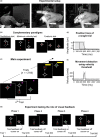
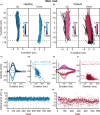

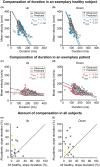
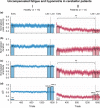

References
-
- Albus, J. S. (1971). A theory of cerebellar function. Mathematical Biosciences, 10, 25–61. 10.1016/0025-5564(71)90051-4 - DOI
-
- Bechert, K. , & Koenig, E. (1996). A search coil system with automatic field stabilization, calibration, and geometric processing for eye movement recording in humans. Neuro‐Ophthalmology, 16, 163–170. 10.3109/01658109609009677 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

