Regulation of Breast Cancer-Induced Osteoclastogenesis by MacroH2A1.2 Involving EZH2-Mediated H3K27me3
- PMID: 29972783
- PMCID: PMC6061927
- DOI: 10.1016/j.celrep.2018.06.020
Regulation of Breast Cancer-Induced Osteoclastogenesis by MacroH2A1.2 Involving EZH2-Mediated H3K27me3
Abstract
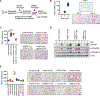
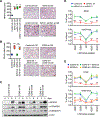
Breast cancer cells relocate to bone and activate osteoclast-induced bone resorption. Soluble factors secreted by breast cancer cells trigger a cascade of events that stimulate osteoclast differentiation in the bone microenvironment. MacroH2A is a unique histone variant with a C-terminal non-histone domain and plays a crucial role in modulating chromatin organization and gene transcription. Here, we show that macroH2A1.2, one of the macroH2A isoforms, has an intrinsic ability to inhibit breast cancer-derived osteoclastogenesis. This repressive effect requires macroH2A1.2-dependent attenuation of expression and secretion of lysyl oxidase (LOX) in breast cancer cells. Furthermore, our mechanistic studies reveal that macroH2A1.2 physically and functionally interacts with the histone methyltransferase EZH2 and elevates H3K27me3 levels to keep LOX gene in a repressed state. Collectively, this study unravels a role for macroH2A1.2 in regulating osteoclastogenic potential of breast cancer cells, suggesting possibilities for developing therapeutic tools to treat osteolytic bone destruction.
Keywords: EZH2; LOX; Src; bone; breast cancer; histone; macroH2A; osteoclast.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures





Similar articles
-
MacroH2A1.2 inhibits prostate cancer-induced osteoclastogenesis through cooperation with HP1α and H1.2.Oncogene. 2018 Oct;37(43):5749-5765. doi: 10.1038/s41388-018-0356-3. Epub 2018 Jun 20. Oncogene. 2018. PMID: 29925860 Free PMC article.
-
Regulation of EZH2 by SMYD2-Mediated Lysine Methylation Is Implicated in Tumorigenesis.Cell Rep. 2019 Nov 5;29(6):1482-1498.e4. doi: 10.1016/j.celrep.2019.10.004. Cell Rep. 2019. PMID: 31693890
-
Noncanonical Functions of the Polycomb Group Protein EZH2 in Breast Cancer.Am J Pathol. 2021 May;191(5):774-783. doi: 10.1016/j.ajpath.2021.01.013. Epub 2021 Feb 6. Am J Pathol. 2021. PMID: 33556366 Free PMC article. Review.
-
Enhancer of zeste homolog 2-catalysed H3K27 trimethylation plays a key role in acute-on-chronic liver failure via TNF-mediated pathway.Cell Death Dis. 2018 May 22;9(6):590. doi: 10.1038/s41419-018-0670-2. Cell Death Dis. 2018. PMID: 29789597 Free PMC article.
-
Evolution, structure and function of divergent macroH2A1 splice isoforms.Semin Cell Dev Biol. 2023 Feb 15;135:43-49. doi: 10.1016/j.semcdb.2022.03.036. Epub 2022 Apr 11. Semin Cell Dev Biol. 2023. PMID: 35422391 Review.
Cited by
-
NCBP3/SNHG6 inhibits GBX2 transcription in a histone modification manner to facilitate the malignant biological behaviour of glioma cells.RNA Biol. 2021 Jan;18(1):47-63. doi: 10.1080/15476286.2020.1790140. Epub 2020 Jul 26. RNA Biol. 2021. PMID: 32618493 Free PMC article.
-
TMBIM6 deficiency leads to bone loss by accelerating osteoclastogenesis.Redox Biol. 2023 Aug;64:102804. doi: 10.1016/j.redox.2023.102804. Epub 2023 Jun 28. Redox Biol. 2023. PMID: 37399733 Free PMC article.
-
DNMT and HDAC inhibitors modulate MMP-9-dependent H3 N-terminal tail proteolysis and osteoclastogenesis.Epigenetics Chromatin. 2019 Apr 16;12(1):25. doi: 10.1186/s13072-019-0270-0. Epigenetics Chromatin. 2019. PMID: 30992059 Free PMC article.
-
MacroH2A1 Immunoexpression in Breast Cancer.Front Oncol. 2020 Aug 21;10:1519. doi: 10.3389/fonc.2020.01519. eCollection 2020. Front Oncol. 2020. PMID: 32974186 Free PMC article.
-
Skeletal Aging and Osteoporosis: Mechanisms and Therapeutics.Int J Mol Sci. 2021 Mar 29;22(7):3553. doi: 10.3390/ijms22073553. Int J Mol Sci. 2021. PMID: 33805567 Free PMC article. Review.
References
-
- Bild AH, Potti A, and Nevins JR (2006). Linking oncogenic pathways with therapeutic opportunities. Nat. Rev. Cancer 6, 735–741. - PubMed
-
- Boyle WJ, Simonet WS, and Lacey DL (2003). Osteoclast differentiation and activation. Nature 423, 337–342. - PubMed
-
- Buschbeck M, Uribesalgo I, Wibowo I, Rué P, Martin D, Gutierrez A, Morey L, Guigo R, Lopez-Schier H, and Di Croce L (2009). The histone variant macroH2A is an epigenetic regulator of key developmental genes. Nat. Struct. Mol. Biol 16, 1074–1079. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

