The Structural Properties in Solution of the Intrinsically Mixed Folded Protein Ataxin-3
- PMID: 29972812
- PMCID: PMC6037153
- DOI: 10.1016/j.bpj.2018.05.029
The Structural Properties in Solution of the Intrinsically Mixed Folded Protein Ataxin-3
Abstract
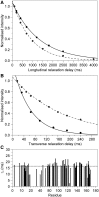
It has increasingly become clear over the last two decades that proteins can contain both globular domains and intrinsically unfolded regions that can both contribute to function. Although equally interesting, the disordered regions are difficult to study, because they usually do not crystallize unless bound to partners and are not easily amenable to cryo-electron microscopy studies. NMR spectroscopy remains the best technique to capture the structural features of intrinsically mixed folded proteins and describe their dynamics. These studies rely on the successful assignment of the spectrum, a task not easy per se given the limited spread of the resonances of the disordered residues. Here, we describe the structural properties of ataxin-3, the protein responsible for the neurodegenerative Machado-Joseph disease. Ataxin-3 is a 42-kDa protein containing a globular N-terminal Josephin domain and a C-terminal tail that comprises 13 polyglutamine repeats within a low complexity region. We developed a strategy that allowed us to achieve 87% assignment of the NMR spectrum using a mixed protocol based on high-dimensionality, high-resolution experiments and different labeling schemes. Thanks to the almost complete spectral assignment, we proved that the C-terminal tail is flexible, with extended helical regions, and interacts only marginally with the rest of the protein. We could also, for the first time to our knowledge, observe the structural propensity of the polyglutamine repeats within the context of the full-length protein and show that its structure is stabilized by the preceding region.
Crown Copyright © 2018. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Paulson H.L., Perez M.K., Pittman R.N. Intranuclear inclusions of expanded polyglutamine protein in spinocerebellar ataxia type 3. Neuron. 1997;19:333–344. - PubMed
-
- Ellisdon A.M., Thomas B., Bottomley S.P. The two-stage pathway of ataxin-3 fibrillogenesis involves a polyglutamine-independent step. J. Biol. Chem. 2006;281:16888–16896. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

