The translational regulation of maternal mRNAs in time and space
- PMID: 29972882
- PMCID: PMC6175449
- DOI: 10.1002/1873-3468.13183
The translational regulation of maternal mRNAs in time and space
Abstract
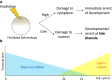
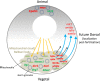
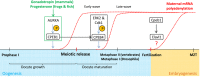
Since their discovery, the study of maternal mRNAs has led to the identification of mechanisms underlying their spatiotemporal regulation within the context of oogenesis and early embryogenesis. Following synthesis in the oocyte, maternal mRNAs are translationally silenced and sequestered into storage in cytoplasmic granules. At the same time, their unique distribution patterns throughout the oocyte and embryo are tightly controlled and connected to their functions in downstream embryonic processes. At certain points in oogenesis and early embryogenesis, maternal mRNAs are translationally activated to perform their functions in a timely manner. The cytoplasmic polyadenylation machinery is responsible for the translational activation of maternal mRNAs, and its role in initiating the maternal to zygotic transition events has recently come to light. Here, we summarize the current knowledge on maternal mRNA regulation, with particular focus on cytoplasmic polyadenylation as a mechanism for translational regulation.
Keywords: cytoplasmic polyadenylation; maternal mRNA; maternal to zygotic transition; translational regulation.
© 2018 The Authors. FEBS Letters published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures
References
-
- Neyfakh AA (1964) Radiation investigation of nucleo‐cytoplasmic interrelations in morphogenesis and biochemical differentiation. Nature 201, 880–884. - PubMed
-
- Neyfakh AA (1961) Comparative study of the morphogenetic function of Nuclei in Animal. Zh Obshch Biol 22, 42–57. - PubMed
-
- Moore JA (1946) Studies in the development of frog hybrids; embryonic development in the cross Rana pipiens female x Rana sylvatica male. J Exp Zool 101, 173–219. - PubMed
-
- Moore JA (1955) Abnormal combinations of nuclear and cytoplasmic systems in frogs and toads. Adv Genet 7, 139–182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

