Predictors of high-cost hospitalization in the treatment of acute coronary syndrome in Asia: findings from EPICOR Asia
- PMID: 29973147
- PMCID: PMC6033225
- DOI: 10.1186/s12872-018-0859-4
Predictors of high-cost hospitalization in the treatment of acute coronary syndrome in Asia: findings from EPICOR Asia
Abstract
Background: The EPICOR Asia (long-tErm follow-uP of antithrombotic management patterns In acute CORonary syndrome patients in Asia) study (NCT01361386) was an observational study of patients hospitalized for acute coronary syndromes (ACS) enrolled in 218 hospitals in eight countries/regions in Asia. This study examined costs, length of stay and the predictors of high costs during an ACS hospitalization.
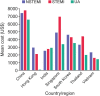
Methods and results: Data for patients hospitalized for an ACS (n = 12,922) were collected on demographics, medical history, event characteristics, socioeconomic and insurance status at discharge. Patients were followed up at 6 weeks' post-hospitalization for an ACS event to assess associated treatment costs from a health sector perspective. Primary outcome was the incurring of costs in the highest quintile by country and index event diagnosis, and identification of associated predictors. Cost data were available for 10,819 patients. Mean length of stay was 10.1 days. The highest-cost countries were China, Singapore, and South Korea. Significant predictors of high-cost care were age, male sex, income, country, prior disease history, hospitalization in 3 months before index event, no dependency before index event, having an invasive procedure, hospital type and length of stay.
Conclusions: Substantial variability exists in healthcare costs for hospitalized ACS patients across Asia. Of concern is the observation that the highest costs were reported in China, given the rapidly increasing numbers of procedures in recent years.
Trial registration: NCT01361386 .
Keywords: Acute coronary syndrome; Asia; Costs; Health insurance; Hospitalization.
Conflict of interest statement
Ethics approval and consent to participate
The study was conducted in compliance with the principles of the Declaration of Helsinki, International Conference on Harmonization Good Clinical Practice guidelines and applicable legislation on non-interventional studies in participating countries and regions. The protocol, including the informed consent form, was approved in writing by the applicable ethics committee of the participating centers in accordance with local regulations in each country. The ethics committee also approved any other non-interventional study documents in accordance with local regulations. Patients provided written informed consent at discharge and completed a contact order form agreeing to be contacted for regular follow-up interviews post discharge.
Consent for publication
Not applicable.
Competing interests
JPSS has been a consultant or advisory board member for AstraZeneca, Lupin, and Intas. TKO has acted as a consultant or advisory board member for Sanofi-Aventis, Abbott Vascular, Boston Scientific, Boehringer Ingelheim, Novartis, and AstraZeneca. CTC has received research support from Eli Lilly, honoraria from Medtronic, and has been a consultant or advisory board member for AstraZeneca. RK has been a consultant or advisory board member for AstraZeneca and Boehringer Ingelheim. VTN has received research grants from AstraZeneca, Servier, Sanofi, and Boston Scientific, and has been a consultant or advisory board member for AstraZeneca, Pfizer, Sanofi, Boehringer Ingelheim, Servier, MSD, Abbott, Bayer, Novartis, Merck Serono, Biosensor, Biotronic, Boston Scientific, Terumo, and Medtronic. SJP receives research funds from AstraZeneca. AMV and NH are employees of AstraZeneca. All other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Wieser S, Ruthemann I, De Boni S, Eichler K, Pletscher M, Radovanovic D, et al. Cost of acute coronary syndrome in Switzerland in 2008. Swiss Med Wkly. 2012;142:w13655. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical