The first next-generation sequencing approach to the mitochondrial phylogeny of African monogenean parasites (Platyhelminthes: Gyrodactylidae and Dactylogyridae)
- PMID: 29973152
- PMCID: PMC6032552
- DOI: 10.1186/s12864-018-4893-5
The first next-generation sequencing approach to the mitochondrial phylogeny of African monogenean parasites (Platyhelminthes: Gyrodactylidae and Dactylogyridae)
Abstract
Background: Monogenean flatworms are the main ectoparasites of fishes. Representatives of the species-rich families Gyrodactylidae and Dactylogyridae, especially those infecting cichlid fishes and clariid catfishes, are important parasites in African aquaculture, even more so due to the massive anthropogenic translocation of their hosts worldwide. Several questions on their evolution, such as the phylogenetic position of Macrogyrodactylus and the highly speciose Gyrodactylus, remain unresolved with available molecular markers. Also, diagnostics and population-level research would benefit from the development of higher-resolution genetic markers. We aim to offer genetic resources for work on African monogeneans by providing mitogenomic data of four species (two belonging to Gyrodactylidae, two to Dactylogyridae), and analysing their gene sequences and gene order from a phylogenetic perspective.
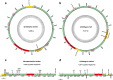
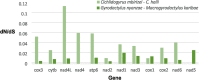
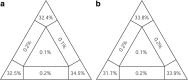
Results: Using Illumina technology, the first four mitochondrial genomes of African monogeneans were assembled and annotated for the cichlid parasites Gyrodactylus nyanzae, Cichlidogyrus halli, Cichlidogyrus mbirizei (near-complete mitogenome) and the catfish parasite Macrogyrodactylus karibae (near-complete mitogenome). Complete nuclear ribosomal operons were also retrieved, as molecular vouchers. The start codon TTG is new for Gyrodactylus and for Dactylogyridae, as is the incomplete stop codon TA for Dactylogyridae. Especially the nad2 gene is promising for primer development. Gene order was identical for protein-coding genes and differed between the African representatives of these families only in a tRNA gene transposition. A mitochondrial phylogeny based on an alignment of nearly 12,500 bp including 12 protein-coding and two ribosomal RNA genes confirms that the Neotropical oviparous Aglaiogyrodactylus forficulatus takes a sister group position with respect to the other gyrodactylids, instead of the supposedly 'primitive' African Macrogyrodactylus. Inclusion of the African Gyrodactylus nyanzae confirms the paraphyly of Gyrodactylus. The position of the African dactylogyrid Cichlidogyrus is unresolved, although gene order suggests it is closely related to marine ancyrocephalines.
Conclusions: The amount of mitogenomic data available for gyrodactylids and dactylogyrids is increased by roughly one-third. Our study underscores the potential of mitochondrial genes and gene order in flatworm phylogenetics, and of next-generation sequencing for marker development for these non-model helminths for which few primers are available.
Keywords: Cichlidae; Cichlidogyrus; Clariidae; Gene order; Gyrodactylus; Macrogyrodactylus; Mitogenome; Monogenea; Monopisthocotylea; Phylogenomics.
Conflict of interest statement
Ethics approval and consent to participate
In the absence of relevant animal welfare regulations in the D.R. Congo, the same strict codes of practice enforced within the European Union were applied. Sampling was carried out under research permit no. 863/2014 from the Faculté des Sciences Agronomiques of the Université de Lubumbashi. Since this research did not involve human subjects, human material, or human data, consent to participate did not apply.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Pugachev ON, Gerasev PI, Gussev AV, Ergens R, Khotenowsky I. Guide to Monogenoidea of freshwater fish of Palaearctic and Amur regions. Milan: Ledizione-LediPublishing; 2009.
-
- Zahradníčková P, Barson M, Luus-Powell WJ, Přikrylová I. Species of Gyrodactylus Von Nordmann, 1832 (Platyhelminthes: Monogenea) from cichlids from Zambezi and Limpopo river basins in Zimbabwe and South Africa: evidence for unexplored species richness. Syst Parasitol. 2016;93:679–700. doi: 10.1007/s11230-016-9652-x. - DOI - PubMed
-
- Paladini G, Longshaw M, Gustinelli A, Shinn AP. Parasitic diseases in aquaculture: their biology, diagnosis and control. In: Austin B, Newaj-Fyzul A, editors. Diagnosis and control of diseases of fish and shellfish. Chichester: John Wiley & Sons, Ltd; 2017. pp. 37–107.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

