Interleukin-1 beta promotes neuronal differentiation through the Wnt5a/RhoA/JNK pathway in cortical neural precursor cells
- PMID: 29973222
- PMCID: PMC6033214
- DOI: 10.1186/s13041-018-0383-6
Interleukin-1 beta promotes neuronal differentiation through the Wnt5a/RhoA/JNK pathway in cortical neural precursor cells
Abstract
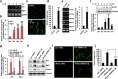
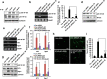
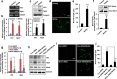
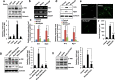
Pro-inflammatory cytokine interleukin-1 beta (IL-1β) is a key mediator of inflammation and stress in the central nervous system (CNS), and is highly expressed in the developing brain. In this study, we investigated the possible role of IL-1β in neuronal differentiation of cortical neural precursor cells (NPCs). We showed that stimulation with IL-1β increased expression levels of neurotrophin-3 (NT3) and neurogenin 1 (Ngn1) and promoted neurite outgrowth. We also found that IL-1β increased mRNA and protein levels of Wnt5a. Knockdown of Wnt5a by transfection with Wnt5a siRNA inhibited IL-1β-induced neuronal differentiation. Moreover, IL-1β-induced Wnt5a expression was regulated by nuclear factor kappa B (NF-κB) activation, which is involved in IL-1β-mediated neuronal differentiation. To examine the role of Wnt5a in neuronal differentiation of NPCs, we exogenously added Wnt5a. We found that exogenous Wnt5a promotes neuronal differentiation, and activates the RhoA/Rho-associated kinase (ROCK)/c-jun N-terminal kinase (JNK) pathway. In addition, Wnt5a-induced neuronal differentiation was blocked by RhoA siRNA, as well as by a specific Rho-kinase inhibitor (Y27632) or a SAPK/JNK inhibitor (SP600125). Furthermore, treatment with RhoA siRNA, Y27632, or SP600125 suppressed the IL-1β-induced neuronal differentiation. Therefore, these results suggest that the sequential Wnt5a/RhoA/ROCK/JNK pathway is involved in IL-1β-induced neuronal differentiation of NPCs.
Keywords: C-jun N-terminal kinase (JNK); Interleukin-1 beta (IL-1β); Neuronal differentiation; RhoA; Wnt5a.
Conflict of interest statement
Ethics approval
All experimental animal procedures (Sprague-Dawley rats) were approved by the Institutional Animal Care and Use Committee (IACUC) at Hanyang College of Medicine under approval number HY-IACUC-17-0035. Experiments were performed in accordance with the NIH guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
The emerging role of Wnt5a in the promotion of a pro-inflammatory and immunosuppressive tumor microenvironment.Cancer Metastasis Rev. 2020 Sep;39(3):933-952. doi: 10.1007/s10555-020-09878-7. Cancer Metastasis Rev. 2020. PMID: 32435939 Review.
-
Phospholipase D1 is an important regulator of bFGF-induced neurotrophin-3 expression and neurite outgrowth in H19-7 cells.Mol Neurobiol. 2012 Jun;45(3):507-19. doi: 10.1007/s12035-012-8268-7. Epub 2012 Apr 28. Mol Neurobiol. 2012. PMID: 22544632
-
Involvement of nuclear factor-kB activation through RhoA/Rho-kinase pathway in LPS-induced IL-8 production in human cervical stromal cells.Mol Hum Reprod. 2007 Mar;13(3):181-7. doi: 10.1093/molehr/gal113. Epub 2007 Jan 16. Mol Hum Reprod. 2007. PMID: 17227815
-
Interleukin-1beta mediates proliferation and differentiation of multipotent neural precursor cells through the activation of SAPK/JNK pathway.Mol Cell Neurosci. 2007 Nov;36(3):343-54. doi: 10.1016/j.mcn.2007.07.005. Epub 2007 Jul 24. Mol Cell Neurosci. 2007. PMID: 17822921
-
JNK signaling and its impact on neural cell maturation and differentiation.Life Sci. 2024 Aug 1;350:122750. doi: 10.1016/j.lfs.2024.122750. Epub 2024 May 25. Life Sci. 2024. PMID: 38801982 Review.
Cited by
-
Salt causes aging-associated hypertension via vascular Wnt5a under Klotho deficiency.J Clin Invest. 2020 Aug 3;130(8):4152-4166. doi: 10.1172/JCI134431. J Clin Invest. 2020. PMID: 32597829 Free PMC article.
-
When the BBB goes MIA.Proc Natl Acad Sci U S A. 2022 May 10;119(19):e2204159119. doi: 10.1073/pnas.2204159119. Epub 2022 May 4. Proc Natl Acad Sci U S A. 2022. PMID: 35507877 Free PMC article. No abstract available.
-
Transcriptional and functional effects of lithium in bipolar disorder iPSC-derived cortical spheroids.Mol Psychiatry. 2023 Jul;28(7):3033-3043. doi: 10.1038/s41380-023-01944-0. Epub 2023 Jan 18. Mol Psychiatry. 2023. PMID: 36653674 Free PMC article.
-
The emerging role of Wnt5a in the promotion of a pro-inflammatory and immunosuppressive tumor microenvironment.Cancer Metastasis Rev. 2020 Sep;39(3):933-952. doi: 10.1007/s10555-020-09878-7. Cancer Metastasis Rev. 2020. PMID: 32435939 Review.
-
Innate immunity at the crossroads of healthy brain maturation and neurodevelopmental disorders.Nat Rev Immunol. 2021 Jul;21(7):454-468. doi: 10.1038/s41577-020-00487-7. Epub 2021 Jan 21. Nat Rev Immunol. 2021. PMID: 33479477 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

