The occurrence, types, consequences and preventability of in-hospital adverse events - a scoping review
- PMID: 29973258
- PMCID: PMC6032777
- DOI: 10.1186/s12913-018-3335-z
The occurrence, types, consequences and preventability of in-hospital adverse events - a scoping review
Abstract
Background: Adverse events (AEs) seriously affect patient safety and quality of care, and remain a pressing global issue. This study had three objectives: (1) to describe the proportions of patients affected by in-hospital AEs; (2) to explore the types and consequences of observed AEs; and (3) to estimate the preventability of in-hospital AEs.
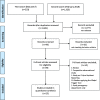
Methods: We applied a scoping review method and concluded a comprehensive literature search in PubMed and CINAHL in May 2017 and in February 2018. Our target was retrospective medical record review studies applying the Harvard method-or similar methods using screening criteria-conducted in acute care hospital settings on adult patients (≥18 years).
Results: We included a total of 25 studies conducted in 27 countries across six continents. Overall, a median of 10% patients were affected by at least one AE (range: 2.9-21.9%), with a median of 7.3% (range: 0.6-30%) of AEs being fatal. Between 34.3 and 83% of AEs were considered preventable (median: 51.2%). The three most common types of AEs reported in the included studies were operative/surgical related, medication or drug/fluid related, and healthcare-associated infections.
Conclusions: Evidence regarding the occurrence of AEs confirms earlier estimates that a tenth of inpatient stays include adverse events, half of which are preventable. However, the incidence of in-hospital AEs varied considerably across studies, indicating methodological and contextual variations regarding this type of retrospective chart review across health care systems. For the future, automated methods for identifying AE using electronic health records have the potential to overcome various methodological issues and biases related to retrospective medical record review studies and to provide accurate data on their occurrence.
Keywords: Adverse events; Hospitals; Medical error; Patient safety; Scoping review.
Conflict of interest statement
Competing interests
All authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- Taylor-Adams S, Vincent C. Systems analysis of clinical incidents: the London protocol. Clinical Risk. 2004;10(6):211–220. doi: 10.1258/1356262042368255. - DOI
-
- U.S. Food and Drug Administration. Reporting serious problems to FDA. https://www.fda.gov/MedicalDevices/default.htm. Accessed 2 July 2018.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources