Screening, purification and characterization of cellulase from cellulase producing bacteria in molasses
- PMID: 29973263
- PMCID: PMC6032522
- DOI: 10.1186/s13104-018-3558-4
Screening, purification and characterization of cellulase from cellulase producing bacteria in molasses
Abstract
Objectives: This study was conducted to isolate, screening and purification of cellulase from bacteria present in sugar industry waste (molasses) and characterization by morphological and biochemical analysis.
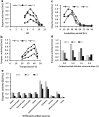
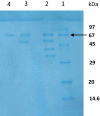
Results: Based on experiments, three bacterial strains produced clear transparent zone into carboxymethyl cellulose (CMC) agar plate were identified as cellulase producing bacteria. Different culture parameters such as pH, temperature, incubation period, substrate concentration and carbon sources were optimized for enzyme production. According to the morphological and biochemical tests, the isolated strains were identified as Paenibacillus sp., Bacillus sp. and Aeromonas sp. The first strain Paenibacillus sp. showed high potentiality for maximum cellulase production (0.9 µmol ml-1 min-1) at pH 7.0 after 24 h of incubation at 40 °C in a medium containing 1.0% CMC. Then Paenibacillus sp. was selected for enzyme purification by ammonium sulfate precipitation, DEAE-cellulose and CM-cellulose column chromatography, respectively. In last step of purification, specific activity, recovery and purification fold were 2655 U/mg, 35.7% and 9.7, respectively. The molecular weight of the purified cellulase was found to be 67 kDa by SDS-PAGE, had an optimal pH and temperature at 7.0 and 40 °C. According to substrate specificity, the purified cellulase had high specificity on CMC substrate which indicated it to be an endo-β-1,4-glucanase.
Keywords: Bacteria; Cellulase; Cellulose; Molasses; Purification and characterization; Screening.
Figures
References
-
- Romeo T. Bacterial biofilms. Berlin: Springer; 2008. pp. 258–263.
-
- Saha S, Roy R, Sen SK, Ray AK. Characterization of cellulase-producing bacteria from the digestive tract of tilapia, Oreochromis mossambica (Peters) and grass carp, Ctenopharyngodon idella (Valenciennes) Aquac Res. 2006;37:380–388. doi: 10.1111/j.1365-2109.2006.01442.x. - DOI
-
- Shanmugapriya K, Saravana PS, Krishnapriya MM, Mythili A, Joseph S. Isolation, screening and partial purification of cellulose from cellulose producing bacteria. Int J Adv Biotechnol Res. 2012;3:509–514.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

