An improvised one-step sucrose cushion ultracentrifugation method for exosome isolation from culture supernatants of mesenchymal stem cells
- PMID: 29973270
- PMCID: PMC6033286
- DOI: 10.1186/s13287-018-0923-0
An improvised one-step sucrose cushion ultracentrifugation method for exosome isolation from culture supernatants of mesenchymal stem cells
Abstract
Background: Exosomes are nanovesicles (30-120 nm) of endosomal origin. These exosomes contain various functional proteins and RNAs that could be used for therapeutic purposes. Currently, having a standard method for exosome isolation retaining its biological properties with increased yield and purity is a major challenge. The most commonly used method is differential ultracentrifugation but it has its own disadvantages, which include high time consumption, low yield due to disruption of exosome integrity, and high protein contaminants. In this study, we have identified an improved method addressing these problems for exosome isolation using ultracentrifugation since it is cost-effective and used worldwide.
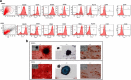
Method: We have compared differential ultracentrifugation with the modified method called one-step sucrose cushion ultracentrifugation for exosome isolation. The conditioned serum-free media from human mesenchymal stem cells cultured for 48 h was collected for exosome isolation. The cellular debris was removed by centrifugation at 300g for 10 min, followed by centrifugation at 10,000g for 30 min to remove microvesicles. Equal volumes of pre-processed conditioned media were used for exosome isolation by direct ultracentrifugation and one-step sucrose cushion ultracentrifugation. The exosomes isolated using these methods were characterized for their size, morphology, concentration, and surface marker protein expression.
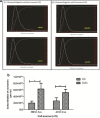
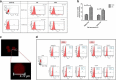
Result: It was observed that the recovery of exosomes with cup-shaped morphology from one-step sucrose cushion ultracentrifugation was comparatively high as estimated by nanoparticle tracking analysis and electron microscopy. These results were confirmed by Western blotting and flow cytometry.
Conclusion: We conclude that this one-step sucrose cushion ultracentrifugation method provides an effective and reproducible potential standard method which could be used for various starting materials for isolating exosomes. We believe that this method will have a wide application in the field of extracellular vesicle research where exosome isolation with high yield and purity is an imperative step. Schematic representation of comparison of UC and SUC exosome isolation methods for tissue-specific human mesenchymal stem cells. The SUC isolation method yields a greater number of cup-shaped exosomes with a relatively homogenous population for mass-scale production of exosomes for downstream analysis.
Abbreviations: SUC One-step sucrose cushion ultracentrifugation, UC Direct ultracentrifugation.
Keywords: Exosomes; Mesenchymal stem cells; Sucrose cushion ultracentrifugation.
Conflict of interest statement
Ethics approval and consent to participate
After approval was obtained from the Institutional Committee for Stem Cell Research, All India Institute of Medical Science, New Delhi (ref. no. ICSCR/34/15(R)), the study was initiated. In this study, cryopreserved BMSCs and ADSCs were used. All methods were performed in accordance with relevant guidelines and regulations of this committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Development of a rinsing separation method for exosome isolation and comparison to conventional methods.Eur Rev Med Pharmacol Sci. 2019 Jun;23(12):5074-5083. doi: 10.26355/eurrev_201906_18171. Eur Rev Med Pharmacol Sci. 2019. PMID: 31298362
-
Exosome-like vesicles in uterine aspirates: a comparison of ultracentrifugation-based isolation protocols.J Transl Med. 2016 Jun 18;14(1):180. doi: 10.1186/s12967-016-0935-4. J Transl Med. 2016. PMID: 27317346 Free PMC article.
-
A feasible method for the isolation of mesenchymal stem cells from menstrual blood and their exosomes.Tissue Cell. 2018 Dec;55:53-62. doi: 10.1016/j.tice.2018.09.010. Epub 2018 Oct 24. Tissue Cell. 2018. PMID: 30503060
-
[Exosome separation and enrichment technologies and their applications in disease diagnosis and treatment].Se Pu. 2025 May;43(5):434-445. doi: 10.3724/SP.J.1123.2024.09007. Se Pu. 2025. PMID: 40331608 Free PMC article. Review. Chinese.
-
A review on comparative studies addressing exosome isolation methods from body fluids.Anal Bioanal Chem. 2023 Mar;415(7):1239-1263. doi: 10.1007/s00216-022-04174-5. Epub 2022 Jul 15. Anal Bioanal Chem. 2023. PMID: 35838769 Review.
Cited by
-
Peptide-based capture-and-release purification of extracellular vesicles and statistical algorithm enabled quality assessment.bioRxiv [Preprint]. 2024 Feb 8:2024.02.06.578050. doi: 10.1101/2024.02.06.578050. bioRxiv. 2024. PMID: 38370748 Free PMC article. Preprint.
-
Emerging Potential of Exosomal Non-coding RNA in Parkinson's Disease: A Review.Front Aging Neurosci. 2022 Mar 10;14:819836. doi: 10.3389/fnagi.2022.819836. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35360206 Free PMC article. Review.
-
The Adaptation of Botrytis cinerea Extracellular Vesicles Proteome to Surrounding Conditions: Revealing New Tools for Its Infection Process.J Fungi (Basel). 2023 Aug 24;9(9):872. doi: 10.3390/jof9090872. J Fungi (Basel). 2023. PMID: 37754980 Free PMC article.
-
Evaluation of exosomal non-coding RNAs in cancer using high-throughput sequencing.J Transl Med. 2022 Jan 15;20(1):30. doi: 10.1186/s12967-022-03231-y. J Transl Med. 2022. PMID: 35033106 Free PMC article. Review.
-
R-loops trigger the release of cytoplasmic ssDNAs leading to chronic inflammation upon DNA damage.Sci Adv. 2021 Nov 19;7(47):eabj5769. doi: 10.1126/sciadv.abj5769. Epub 2021 Nov 19. Sci Adv. 2021. PMID: 34797720 Free PMC article.
References
-
- Singh M, Gupta S, Rawat S, Midha S, Jain KG, Dalela M, Mohanty S. Mechanisms of Action of Human Mesenchymal Stem Cells in Tissue Repair Regeneration and Their Implications. Ann Natl Acad Med Sci (India) 2017;53(2):104–120.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

