The age of onset and evolution of Braak tangle stage and Thal amyloid pathology of Alzheimer's disease in individuals with Down syndrome
- PMID: 29973279
- PMCID: PMC6030772
- DOI: 10.1186/s40478-018-0559-4
The age of onset and evolution of Braak tangle stage and Thal amyloid pathology of Alzheimer's disease in individuals with Down syndrome
Abstract
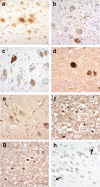
While post mortem studies have identified the major cell types and functional systems affected in Alzheimer's disease (AD) the initial sites and molecular characteristics of pathology are still unclear. Because individuals with Down syndrome (DS) (trisomy 21) develop the full pathological changes of AD in a predictable way by the time they reach middle to late age, a study of the brains of such persons at different ages makes an ideal 'model system' in which the sites of earliest onset of pathology can be detected and the subsequent progression of changes be monitored. In the present study we have examined the brains of 56 individuals with DS ranging from new-born to 76 years for the presence of amyloid and tau pathology in key cortical and subcortical regions. Amyloid pathology was found to commence in the late teens to twenties as a deposition of diffuse plaques initially within the temporal neocortex, quickly involving other neocortical regions but only reaching subcortical regions and cerebellum by the late forties. Cerebral amyloid angiopathy did not regularly commence until after 45-50 years of age. Tau pathology usually commenced after 35 years of age, initially involving not only entorhinal areas and hippocampus but also subcortical regions such as locus caeruleus (LC) and dorsal raphe nucleus (DRN). Later, tau pathology spread throughout the neocortex reaching occipital lobes in most instances by mid-50 years of age. Such a pattern of spread is consistent with that seen in typical AD. We found no evidence that tau pathology might commence within the brain in DS before amyloid deposition had occurred, but there was limited data that suggests tau pathology in LC or DRN might predate that in entorhinal areas and hippocampus or at least be coincident.
Keywords: Alzheimer’s disease; Amyloid; Down syndrome; Tau.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by Manchester Brain Bank Management Committee (REC reference 09/H0906/52 + 5). Under conditions agreed with the Research Ethics Committee, The Manchester Brain Bank can supply tissue or data to researchers, without requirement for researchers to apply individually to the REC for approval.
Consent for publication
All authors have read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

