Sites of gastrointestinal lesion induced by mycophenolate mofetil: a comparison with enteric-coated mycophenolate sodium in rats
- PMID: 29973291
- PMCID: PMC6030804
- DOI: 10.1186/s40360-018-0234-1
Sites of gastrointestinal lesion induced by mycophenolate mofetil: a comparison with enteric-coated mycophenolate sodium in rats
Abstract
Background: Immunosuppressant drugs for renal transplant mycophenolate mofetil (MMF) and enteric-coated mycophenolate sodium (EC-MPS) cause gastrointestinal (GI) disorders. The specific site of GI tract targeted by MMF and EC-MPS remains unclear.
Methods: In this study, we investigated the effects of MMF and EC-MPS on stomach, duodenum, jejunum, ileum, colon and rectum using a rat model. Rats were randomized into five groups: control, MMF (100 mg/kg·d), mofetil (30 mg/kg·d), EC-MPS (72 mg/Kg·d), mofetil + EC-MPS. Each group was treated with drugs once a day for 7 days through intra-gastric gavage. Diarrhea grade of each rat were measured every day, as well as the body weight. Blood was collected by tail nick and Seven days later, the rats were sacrificed, GI tissues were collected for Histological research.
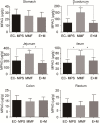
Results: The results showed that diarrhea grade and weight loss were significantly higher in MMF group than other groups. The pathological score of MMF group was significantly higher than EC-MPS group and EC-MPS + mofetil group in jejunum and ileum tissues, but not other segments of GI tract. Absorption of EC-MPS is delayed, compared to that of MMF. MPAG concentration in duodenum, jejunum and ileum tissues of MMF group is higher than EC-MPS group. Mofetil may increase the magnitude of MPA absorption.
Conclusions: Our data suggested that MMF might target jejunum and ileum and induce GI injury. EC-MPS causes less injury in GI tract than MMF, probably due to its kinetic property.
Keywords: Enteric-coated mycophenolate sodium; GI side effects; Mycophenolate mofetil; Pharmacokinetics.
Conflict of interest statement
Ethics approval and consent to participate
The experimental protocol was approved by the Committee of Animal Care of Fudan University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Gastrointestinal quality of life improvement of renal transplant recipients converted from mycophenolate mofetil to enteric-coated mycophenolate sodium drugs or agents: mycophenolate mofetil and enteric-coated mycophenolate sodium.Transplantation. 2011 Aug 27;92(4):426-32. doi: 10.1097/TP.0b013e31822527ca. Transplantation. 2011. PMID: 21760569 Clinical Trial.
-
Better mycophenolic acid 12-hour trough level after enteric-coated mycophenolate sodium in patients with gastrointestinal intolerance to mycophenolate mofetil.Transplant Proc. 2007 Sep;39(7):2194-6. doi: 10.1016/j.transproceed.2007.06.033. Transplant Proc. 2007. PMID: 17889135
-
Comparing outcomes associated with dose manipulations of enteric-coated mycophenolate sodium versus mycophenolate mofetil in renal transplant recipients.Transplantation. 2009 Aug 27;88(4):514-20. doi: 10.1097/TP.0b013e3181b0e65e. Transplantation. 2009. PMID: 19696634
-
Enteric-coated mycophenolate sodium: therapeutic equivalence to mycophenolate mofetil in de novo renal transplant patients.Transplant Proc. 2004 Mar;36(2 Suppl):517S-520S. doi: 10.1016/j.transproceed.2004.01.052. Transplant Proc. 2004. PMID: 15041399 Review.
-
Mycophenolate, clinical pharmacokinetics, formulations, and methods for assessing drug exposure.Transplant Rev (Orlando). 2011 Apr;25(2):47-57. doi: 10.1016/j.trre.2010.06.001. Epub 2010 Dec 28. Transplant Rev (Orlando). 2011. PMID: 21190834
Cited by
-
Development of a novel UPLC-MS/MS method for the simultaneous quantification of mycophenolic mofetil, mycophenolic acid, and its major metabolites: Application to pharmacokinetic and tissue distribution study in rats.J Pharm Biomed Anal. 2023 Sep 20;234:115504. doi: 10.1016/j.jpba.2023.115504. Epub 2023 Jun 3. J Pharm Biomed Anal. 2023. PMID: 37478553 Free PMC article.
-
Giant Gastric Ulcers: An Unusual Culprit.Dig Dis Sci. 2020 Oct;65(10):2811-2817. doi: 10.1007/s10620-020-06573-z. Dig Dis Sci. 2020. PMID: 32875528 Free PMC article.
-
Recent advances in graves ophthalmopathy medical therapy: a comprehensive literature review.Int Ophthalmol. 2023 Apr;43(4):1437-1449. doi: 10.1007/s10792-022-02537-6. Epub 2022 Oct 22. Int Ophthalmol. 2023. PMID: 36272013 Free PMC article. Review.
-
Gut mucosa alterations after kidney transplantation: a cross sectional study.J Nephrol. 2025 May;38(4):1153-1162. doi: 10.1007/s40620-024-02067-7. Epub 2024 Sep 18. J Nephrol. 2025. PMID: 39289297 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

