Iberian pig mesenchymal stem/stromal cells from dermal skin, abdominal and subcutaneous adipose tissues, and peripheral blood: in vitro characterization and migratory properties in inflammation
- PMID: 29973295
- PMCID: PMC6032775
- DOI: 10.1186/s13287-018-0933-y
Iberian pig mesenchymal stem/stromal cells from dermal skin, abdominal and subcutaneous adipose tissues, and peripheral blood: in vitro characterization and migratory properties in inflammation
Abstract
Background: Recently, the capacity of mesenchymal stem/stromal cells (MSCs) to migrate into damaged tissues has been reported. For MSCs to be a promising tool for tissue engineering and cell and gene therapy, it is essential to know their migration ability according to their tissue of origin. However, little is known about the molecular mechanisms regulating porcine MSC chemotaxis. The aim of this study was to examine the migratory properties in an inflammatory environment of porcine MSC lines from different tissue origins: subcutaneous adipose tissue (SCA-MSCs), abdominal adipose tissue (AA-MSCs), dermal skin tissue (DS-MSCs) and peripheral blood (PB-MSCs).
Methods: SCA-MSCs, AA-MSCs, DS-MSCs and PB-MSCs were isolated and analyzed in terms of morphological features, alkaline phosphatase activity, expression of cell surface and intracellular markers of pluripotency, proliferation, in vitro chondrogenic, osteogenic and adipogenic differentiation capacities, as well as their ability to migrate in response to inflammatory cytokines.
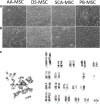
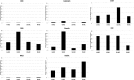
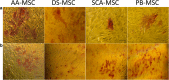
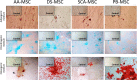
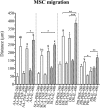
Results: SCA-MSCs, AA-MSCs, DS-MSCs and PB-MSCs were isolated and showed plastic adhesion with a fibroblast-like morphology. All MSC lines were positive for CD44, CD105, CD90 and vimentin, characteristic markers of MSCs. The cytokeratin marker was also detected in DS-MSCs. No expression of MHCII or CD34 was detected in any of the four types of MSC. In terms of pluripotency features, all MSC lines expressed POU5F1 and showed alkaline phosphatase activity. SCA-MSCs had a higher growth rate compared to the rest of the cell lines, while the AA-MSC cell line had a longer population doubling time. All MSC lines cultured under adipogenic, chondrogenic and osteogenic conditions showed differentiation capacity to the previously mentioned mesodermal lineages. All MSC lines showed migration ability in an agarose drop assay. DS-MSCs migrated greater distances than the rest of the cell lines both in nonstimulated conditions and in the presence of the inflammatory cytokines TNF-α and IL-1β. SCA-MSCs and DS-MSCs increased their migration capacity in the presence of IL-1β as compared to PBS control.
Conclusions: This study describes the isolation and characterization of porcine cell lines from different tissue origin, with clear MSC properties. We show for the first time a comparative study of the migration capacity induced by inflammatory mediators of porcine MSCs of different tissue origin.
Keywords: Cell migration; Iberian pig; Inflammation; Mesenchymal stem/stromal cells.
Conflict of interest statement
Ethics approval and consent to participate
All experimental procedures complied with the basic standards for the protection of animals used for experimental and other scientific purposes including teaching, stipulated by Ministry of Agriculture, Food and Environment. The procedures used in animals have an established Animal Use Protocol approved by the Ethics Committee Animal Experimentation at INIA. Animal manipulations were performed according to the Spanish Policy for Animal Protection RD1201/05, which meets the European Union Directive 86/609 about the protection of animals used in research. Tissue samples were taken from an Iberian boar housed in the INIA Animal Laboratory Unit (Madrid, Spain), which meets the requirements of the European Union for Scientific Procedure Establishments.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Bovine endometrial MSC: mesenchymal to epithelial transition during luteolysis and tropism to implantation niche for immunomodulation.Stem Cell Res Ther. 2019 Jan 11;10(1):23. doi: 10.1186/s13287-018-1129-1. Stem Cell Res Ther. 2019. PMID: 30635057 Free PMC article.
-
A Comparative Study of Growth Kinetics, In Vitro Differentiation Potential and Molecular Characterization of Fetal Adnexa Derived Caprine Mesenchymal Stem Cells.PLoS One. 2016 Jun 3;11(6):e0156821. doi: 10.1371/journal.pone.0156821. eCollection 2016. PLoS One. 2016. PMID: 27257959 Free PMC article.
-
Comparison of Biological Features of Wild European Rabbit Mesenchymal Stem Cells Derived from Different Tissues.Int J Mol Sci. 2022 Jun 8;23(12):6420. doi: 10.3390/ijms23126420. Int J Mol Sci. 2022. PMID: 35742872 Free PMC article.
-
Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways.Tissue Eng Part B Rev. 2012 Dec;18(6):436-44. doi: 10.1089/ten.TEB.2012.0014. Epub 2012 Aug 6. Tissue Eng Part B Rev. 2012. PMID: 22741572 Free PMC article. Review.
-
Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches.Eur Cell Mater. 2010 Sep 1;20:121-33. doi: 10.22203/ecm.v020a11. Eur Cell Mater. 2010. PMID: 21249629 Review.
Cited by
-
Cryobanking European Mink (Mustela lutreola) Mesenchymal Stem Cells and Oocytes.Int J Mol Sci. 2022 Aug 18;23(16):9319. doi: 10.3390/ijms23169319. Int J Mol Sci. 2022. PMID: 36012583 Free PMC article.
-
Bovine endometrial MSC: mesenchymal to epithelial transition during luteolysis and tropism to implantation niche for immunomodulation.Stem Cell Res Ther. 2019 Jan 11;10(1):23. doi: 10.1186/s13287-018-1129-1. Stem Cell Res Ther. 2019. PMID: 30635057 Free PMC article.
-
Detrimental alteration of mesenchymal stem cells by an articular inflammatory microenvironment results in deterioration of osteoarthritis.BMC Med. 2023 Jun 19;21(1):215. doi: 10.1186/s12916-023-02923-6. BMC Med. 2023. PMID: 37337188 Free PMC article.
-
Differentiation Potential of Mesenchymal Stem/Stromal Cells Is Altered by Intrauterine Growth Restriction.Front Vet Sci. 2020 Nov 5;7:558905. doi: 10.3389/fvets.2020.558905. eCollection 2020. Front Vet Sci. 2020. PMID: 33251256 Free PMC article.
-
Characteristics and regulation of mesenchymal stem cell plasticity by the microenvironment - specific factors involved in the regulation of MSC plasticity.Genes Dis. 2020 Oct 27;9(2):296-309. doi: 10.1016/j.gendis.2020.10.006. eCollection 2022 Mar. Genes Dis. 2020. PMID: 35224147 Free PMC article. Review.
References
-
- Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–274. - PubMed
-
- Ock SA, Baregundi Subbarao R, Lee YM, Lee JH, Jeon RH, Lee SL, et al. Comparison of immunomodulation properties of porcine mesenchymal stromal/stem cells derived from the bone marrow, adipose tissue, and dermal skin tissue. Stem Cells Int. 2016;2016:9581350. doi: 10.1155/2016/9581350. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

