Cezanne/OTUD7B is a cell cycle-regulated deubiquitinase that antagonizes the degradation of APC/C substrates
- PMID: 29973362
- PMCID: PMC6092620
- DOI: 10.15252/embj.201798701
Cezanne/OTUD7B is a cell cycle-regulated deubiquitinase that antagonizes the degradation of APC/C substrates
Abstract
The anaphase-promoting complex/cyclosome (APC/C) is an E3 ubiquitin ligase and key regulator of cell cycle progression. Since APC/C promotes the degradation of mitotic cyclins, it controls cell cycle-dependent oscillations in cyclin-dependent kinase (CDK) activity. Both CDKs and APC/C control a large number of substrates and are regulated by analogous mechanisms, including cofactor-dependent activation. However, whereas substrate dephosphorylation is known to counteract CDK, it remains largely unknown whether deubiquitinating enzymes (DUBs) antagonize APC/C substrate ubiquitination during mitosis. Here, we demonstrate that Cezanne/OTUD7B is a cell cycle-regulated DUB that opposes the ubiquitination of APC/C targets. Cezanne is remarkably specific for K11-linked ubiquitin chains, which are formed by APC/C in mitosis. Accordingly, Cezanne binds established APC/C substrates and reverses their APC/C-mediated ubiquitination. Cezanne depletion accelerates APC/C substrate degradation and causes errors in mitotic progression and formation of micronuclei. These data highlight the importance of tempered APC/C substrate destruction in maintaining chromosome stability. Furthermore, Cezanne is recurrently amplified and overexpressed in numerous malignancies, suggesting a potential role in genome maintenance and cancer cell proliferation.
Keywords: APC/C; cell cycle; deubiquitinase; mitosis; ubiquitination.
© 2018 The Authors.
Figures

Recombinant GST‐Cezanne (0.2 μM) was incubated with 1 μM of the indicated diubiquitin probes in DUB reaction buffer at room temperature. Aliquots were collected at the indicated time points and analyzed by silver stain.
Recombinant GST‐Cezanne (0.1 μM) was incubated with 1 μM of K11‐linked DiUb or TetraUb in DUB reaction buffer at room temperature. Aliquots were collected at the indicated time points and analyzed by silver stain.
U2OS cells were synchronized in mitosis with nocodazole, isolated by “shake‐off”, and analyzed by immunoblot after release into the cell cycle.
HCT116 cells grown asynchronously or synchronized in mitosis with nocodazole and isolated by “shake‐off” were analyzed by immunoblot with the indicated antibodies.
Representative immunofluorescence images stained for Cezanne, Tubulin, and DNA during the cell cycle in U2OS. Quantification of Cezanne intensity between interphase and mitotic cells is shown on the right (error bars show standard deviation for n = 105 and 28 interphase and mitotic cells, respectively).

RPE1 cells were synchronized by serum deprivation and then released into the cell cycle and analyzed by immunoblot.
U2OS cells were synchronized in mitosis with nocodazole, isolated by “shake‐off”, and released into the cell cycle with or without the proteasomal inhibitor MG132. Samples were analyzed by immunoblot.
U2OS cells grown asynchronously or synchronized in mitosis with nocodazole and isolated by “shake‐off” were analyzed by immunoblot and RT–PCR.

HA‐Cezanne and Myc‐FoxM1 were ectopically expressed in HEK‐293T cells. After 24 h, cells were treated with 20 μM of MG132 for 4 h and Cezanne was immunoprecipitated on anti‐HA beads. Immunoblotted antigen is underlined to the right of blots.
HA‐Cezanne and Myc‐Aurora A interaction was analyzed as in (A), except that no MG132 was added before the IP.
HA‐Cezanne and Venus‐Cyclin B interaction was analyzed as in (B).
GST‐Cezanne was incubated on beads with in vitro‐translated Cyclin B. In vitro binding was analyzed by immunoblot using anti‐Cyclin B antibodies. GST was used as a negative control.
In vitro binding between Cezanne and Aurora A was analyzed as in (C), except that Aurora A was produced in bacteria and detected using anti‐6HIS antibodies.
Lysates of U2OS cells grown asynchronously or synchronized in mitosis with nocodazole were incubated with GST‐Cezanne on beads. GST was used as a negative control and protein detected by immunoblot.
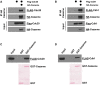
HA‐Cezanne and FLAG‐Cdc20 were ectopically expressed in HEK‐293T cells, and Cezanne was immunoprecipitated on anti‐HA beads. Samples were analyzed by immunoblot with the indicated antibodies.
HA‐Cezanne and FLAG‐Cdh1 interaction was analyzed as in (A).
5 μg of GST‐Cezanne coated on GSH beads was incubated with lysate of HEK‐293T cells expressing a FLAG‐tagged version of Cdc20. In vitro binding was analyzed by immunoblot using the indicated antibodies. GST was used as a negative control.
Interaction of GST‐Cezanne with Cdh1 was analyzed as in (C).
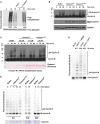
G1 extract from HeLaS3 cells was prepared and mixed with ATP, UBE2C, UBE2S, and fluorescent ubiquitin. Where indicated, recombinant Emi1 or Cezanne was also added. Reactions were incubated at 25°C and analyzed by SDS–PAGE and fluorescence scanning.
G1 extract from HeLaS3 cells was prepared and mixed with ATP, UBE2C, ubiquitin, and either 2 μM of recombinant CezanneWT or CezanneC194S. Reactions were incubated at room temperature and analyzed by SDS–PAGE and immunoblot.
APC/C was immunopurified from mitotic HeLaS3 cell extracts using anti‐Cdc27 beads and then mixed with in vitro‐translated Cyclin B, E1, E2, ATP, ubiquitin, and indicated amounts of Cezanne. Aliquots were collected at the indicated time points and analyzed by immunoblot using Cyclin B antibodies.
Ubiquitination reactions of a fluorescein‐labeled substrate N‐terminal fragment of Cyclin B fused to ubiquitin (Ub‐Cyclin B) were carried out in the presence of either recombinant APC/CCdh1, Cdc34, or Ubc13 and UEV1 to generate the indicated ubiquitin chain topologies. Reactions were quenched with EDTA and then analyzed by SDS–PAGE and fluorescence scanning.
Fluorescein‐labeled Ub‐Cyclin conjugated to K11, K48, or K63 ubiquitin chains was incubated with recombinant Cezanne for the indicated times and then analyzed by SDS–PAGE, fluorescence scanning, and Coomassie staining.

Ubiquitination reactions of a fluorescein‐labeled Securin fused to ubiquitin (Ub‐Securin) were carried out in the presence of either recombinant APC/CCdh1, Cdc34, or Ubc13 and UEV1 to generate the indicated ubiquitin chain topologies. Reactions were quenched with EDTA and then analyzed by SDS–PAGE and fluorescence scanning.
Fluorescein‐labeled Ub‐Securin conjugated to K11, K48, or K63 ubiquitin chains was incubated with recombinant Cezanne for the indicated times and then analyzed by SDS–PAGE, fluorescence scanning, and Coomassie staining.
Fluorescein‐labeled Ub‐Cyclin conjugated to K48 or K63 ubiquitin chains was incubated with recombinant Cezanne in the absence or presence of APCCdh1 for the indicated times and then analyzed by SDS–PAGE, fluorescence scanning, and Coomassie staining.

Degradation assay of endogenous Aurora A using a G1 HeLaS3 cell extract supplemented with ATP, ubiquitin, UBE2C, and Cezanne. Aliquots were collected at the indicated time points and analyzed by immunoblot.
Degradation assay was performed as in (A) except that in vitro‐translated Cyclin B was used as a substrate. Aliquots were collected at the indicated time points and analyzed by immunoblot.
U2OS cells were transfected with the control firefly (FF), Cezanne, and Cezanne with UBE2S siRNAs for 48 h and analyzed by immunoblot with the indicated antibodies.
U2OS cells were analyzed as in (C).
U2OS cells were transfected with the indicated siRNAs, and after 48 h of knock down, an empty vector or an siRNA‐resistant Cezanne vector was introduced by transfection for an additional 24 h. Cells were analyzed by immunoblot.
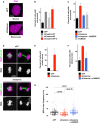
Representative immunofluorescence images of micronuclei in control or Cezanne‐depleted U2OS cells (scale bar = 10 μm).
Quantification of cells with micronuclei for each condition (error bars show standard deviation, duplicate experiments, n > 930 cells per condition).
Quantification of U2OS cells with micronuclei from control cells (black), Cezanne only depleted (red), UBE2S only depleted (gray), or Cezanne and UBE2S co‐depleted cells (blue) (error bars show standard deviation, duplicate experiments, n > 980 cells per condition).
Representative immunofluorescence images of metaphase and anaphase in control or Cezanne‐depleted U2OS cells (scale bar = 10 μm).
Quantification of mitotic error frequency in control or Cezanne‐depleted cells (error bars show standard deviation, duplicate experiments, n > 62 cells per condition).
Quantification of mitotic error frequency in control cells (black), Cezanne only depleted (red), or Cezanne and UBE2S co‐depleted cells (blue) (error bars show standard deviation, duplicate experiments, n > 140 cells per condition).
GFP‐H2B U2OS cells transfected with control (black), Cezanne (red), or Cezanne and UBE2S siRNAs (blue) were subjected to live cell imaging. Cells were then manually tracked from the onset of visible chromosomal condensation in mitosis until late anaphase/early telophase. One hundred and fifty mitotic events were analyzed per siRNA condition, and P‐values between conditions were computed using the non‐parametric two‐sample Wilcoxon test (box and whisker plots represent the distribution of the values to allow visualization of the median, upper, and lower quartiles).
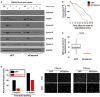
U2OS cells transfected with firefly control or Cezanne siRNA (siFF and siCezanne, respectively) and synchronized in mitosis were released in fresh medium and analyzed at the indicated time points by immunoblot. Additional immunoblotting of these samples is shown in Appendix Fig S4, and the same Cezanne and GAPDH blots are shown in both figures.
Representative in vivo degradation curves of Venus‐Cyclin B during mitosis from control (black) or Cezanne‐depleted cells (red).
Quantification of Venus‐Cyclin B degradation curves from control U2OS cells (black) or Cezanne‐depleted cells (red). Thirty cells per condition were analyzed (box and whisker plots represent the distribution of the values to allow visualization of the median, upper, and lower quartiles—note that the box for the Cezanne plot is not immediately visible because the median and first and third quartiles are all equal to 36. This indicates the narrow distribution of points around the median).
Cell cycle progression was analyzed using on plate EdU labeling in control and Cezanne‐depleted U2OS cells (scale bar = 400 μm).
References
-
- Binné UK, Classon MK, Dick FA, Wei W, Rape M, Kaelin WG, Näär AM, Dyson NJ (2007) Retinoblastoma protein and anaphase‐promoting complex physically interact and functionally cooperate during cell‐cycle exit. Nat Cell Biol 9: 225–232 - PubMed
-
- Bonacci T, Audebert S, Camoin L, Baudelet E, Bidaut G, Garcia M, Witzel II, Perkins ND, Borg JP, Iovanna JL, Soubeyran P (2014) Identification of new mechanisms of cellular response to chemotherapy by tracking changes in post‐translational modifications by ubiquitin and ubiquitin‐like proteins. J Proteome Res 13: 2478–2494 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

