Overview of Antibody Drug Delivery
- PMID: 29973504
- PMCID: PMC6161251
- DOI: 10.3390/pharmaceutics10030083
Overview of Antibody Drug Delivery
Abstract
Monoclonal antibodies (mAbs) are one of the most important classes of therapeutic proteins, which are used to treat a wide number of diseases (e.g., oncology, inflammation and autoimmune diseases). Monoclonal antibody technologies are continuing to evolve to develop medicines with increasingly improved safety profiles, with the identification of new drug targets being one key barrier for new antibody development. There are many opportunities for developing antibody formulations for better patient compliance, cost savings and lifecycle management, e.g., subcutaneous formulations. However, mAb-based medicines also have limitations that impact their clinical use; the most prominent challenges are their short pharmacokinetic properties and stability issues during manufacturing, transport and storage that can lead to aggregation and protein denaturation. The development of long acting protein formulations must maintain protein stability and be able to deliver a large enough dose over a prolonged period. Many strategies are being pursued to improve the formulation and dosage forms of antibodies to improve efficacy and to increase the range of applications for the clinical use of mAbs.
Keywords: antibodies; drug delivery; pharmacokinetics; protein; stability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
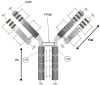
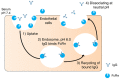
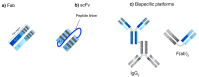

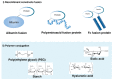
References
-
- Beck A., Wagner-Rousset E., Bussat M.C., Lokteff M., Klinguer-Hamour C., Haeuw J.F., Goetsch L., Wurch T., Dorsselaer A.V., Corvaïa N. Trends in glycosylation, glycoanalysis and glycoengineering of therapeutic antibodies and Fc-fusion proteins. Curr. Pharm. Biotechnol. 2008;9:482–501. doi: 10.2174/138920108786786411. - DOI - PubMed
-
- Mahmuda A., Bande F., Jameel K., Abdulhaleem N., Majid R.A., Hamat R.A., Abdullah W.O., Unyah Z. Monoclonal antibodies: A review of therapeutic applications and future prospects. Trop. J. Pharm. Res. 2017;16:713. doi: 10.4314/tjpr.v16i3.29. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

