The Importance of Multifrequency Impedance Sensing of Endothelial Barrier Formation Using ECIS Technology for the Generation of a Strong and Durable Paracellular Barrier
- PMID: 29973526
- PMCID: PMC6163417
- DOI: 10.3390/bios8030064
The Importance of Multifrequency Impedance Sensing of Endothelial Barrier Formation Using ECIS Technology for the Generation of a Strong and Durable Paracellular Barrier
Abstract
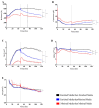
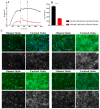
In this paper, we demonstrate the application of electrical cell-substrate impedance sensing (ECIS) technology for measuring differences in the formation of a strong and durable endothelial barrier model. In addition, we highlight the capacity of ECIS technology to model the parameters of the physical barrier associated with (I) the paracellular space (referred to as Rb) and (II) the basal adhesion of the endothelial cells (α, alpha). Physiologically, both parameters are very important for the correct formation of endothelial barriers. ECIS technology is the only commercially available technology that can measure and model these parameters independently of each other, which is important in the context of ascertaining whether a change in overall barrier resistance (R) occurs because of molecular changes in the paracellular junctional molecules or changes in the basal adhesion molecules. Finally, we show that the temporal changes observed in the paracellular Rb can be associated with changes in specific junctional proteins (CD144, ZO-1, and catenins), which have major roles in governing the overall strength of the junctional communication between neighbouring endothelial cells.
Keywords: CD144; ECIS; ZO-1; blood brain barrier; endothelial; resistance.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Reiss B., Wegener J. Impedance analysis of different cell monolayers grown on gold-film electrodes; Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); Milan, Italy. 25–29 August 2015; pp. 7079–7082. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

