Species-specific activity of antibacterial drug combinations
- PMID: 29973719
- PMCID: PMC6219701
- DOI: 10.1038/s41586-018-0278-9
Species-specific activity of antibacterial drug combinations
Abstract
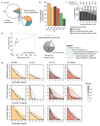
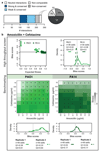
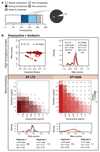
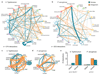
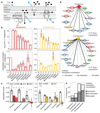
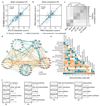
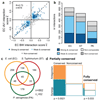
The spread of antimicrobial resistance has become a serious public health concern, making once-treatable diseases deadly again and undermining the achievements of modern medicine1,2. Drug combinations can help to fight multi-drug-resistant bacterial infections, yet they are largely unexplored and rarely used in clinics. Here we profile almost 3,000 dose-resolved combinations of antibiotics, human-targeted drugs and food additives in six strains from three Gram-negative pathogens-Escherichia coli, Salmonella enterica serovar Typhimurium and Pseudomonas aeruginosa-to identify general principles for antibacterial drug combinations and understand their potential. Despite the phylogenetic relatedness of the three species, more than 70% of the drug-drug interactions that we detected are species-specific and 20% display strain specificity, revealing a large potential for narrow-spectrum therapies. Overall, antagonisms are more common than synergies and occur almost exclusively between drugs that target different cellular processes, whereas synergies are more conserved and are enriched in drugs that target the same process. We provide mechanistic insights into this dichotomy and further dissect the interactions of the food additive vanillin. Finally, we demonstrate that several synergies are effective against multi-drug-resistant clinical isolates in vitro and during infections of the larvae of the greater wax moth Galleria mellonella, with one reverting resistance to the last-resort antibiotic colistin.
Figures









References
-
- Draft political declaration of the high-level meeting of the United Nations General Assembly on antimicrobial resistance. 2016 ( http://www.un.org/pga/71/2016/09/21/press-release-hl-meeting-on-antimicr...)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

