Mechanism for remodelling of the cell cycle checkpoint protein MAD2 by the ATPase TRIP13
- PMID: 29973720
- PMCID: PMC6057611
- DOI: 10.1038/s41586-018-0281-1
Mechanism for remodelling of the cell cycle checkpoint protein MAD2 by the ATPase TRIP13
Abstract
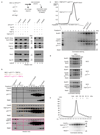
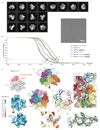
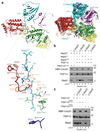
The maintenance of genome stability during mitosis is coordinated by the spindle assembly checkpoint (SAC) through its effector the mitotic checkpoint complex (MCC), an inhibitor of the anaphase-promoting complex (APC/C, also known as the cyclosome)1,2. Unattached kinetochores control MCC assembly by catalysing a change in the topology of the β-sheet of MAD2 (an MCC subunit), thereby generating the active closed MAD2 (C-MAD2) conformer3-5. Disassembly of free MCC, which is required for SAC inactivation and chromosome segregation, is an ATP-dependent process driven by the AAA+ ATPase TRIP13. In combination with p31comet, an SAC antagonist6, TRIP13 remodels C-MAD2 into inactive open MAD2 (O-MAD2)7-10. Here, we present a mechanism that explains how TRIP13-p31comet disassembles the MCC. Cryo-electron microscopy structures of the TRIP13-p31comet-C-MAD2-CDC20 complex reveal that p31comet recruits C-MAD2 to a defined site on the TRIP13 hexameric ring, positioning the N terminus of C-MAD2 (MAD2NT) to insert into the axial pore of TRIP13 and distorting the TRIP13 ring to initiate remodelling. Molecular modelling suggests that by gripping MAD2NT within its axial pore, TRIP13 couples sequential ATP-driven translocation of its hexameric ring along MAD2NT to push upwards on, and simultaneously rotate, the globular domains of the p31comet-C-MAD2 complex. This unwinds a region of the αA helix of C-MAD2 that is required to stabilize the C-MAD2 β-sheet, thus destabilizing C-MAD2 in favour of O-MAD2 and dissociating MAD2 from p31comet. Our study provides insights into how specific substrates are recruited to AAA+ ATPases through adaptor proteins and suggests a model of how translocation through the axial pore of AAA+ ATPases is coupled to protein remodelling.
Conflict of interest statement
Figures











References
-
- Musacchio A. The Molecular Biology of Spindle Assembly Checkpoint Signaling Dynamics. Current biology : CB. 2015;25:R1002–1018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

