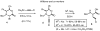Stereocontrolled Synthesis of Quaternary β,γ-Unsaturated Amino Acids: Chain Extension of D- & L- α-(2-Tributylstannyl)Vinyl Amino Acids
- PMID: 29973743
- PMCID: PMC6027620
- DOI: 10.1016/S0040-4020(01)00499-9
Stereocontrolled Synthesis of Quaternary β,γ-Unsaturated Amino Acids: Chain Extension of D- & L- α-(2-Tributylstannyl)Vinyl Amino Acids
Abstract
A pair of diastereomeric (4S,5S)- and (4S,5R)-4-methoxycarbonyl-5-phenylselenomethyl-2-phenyl oxazolines, derived from L-vinylglycine, serve as precursors to protected, quaternary, L- and D-α-(2-tributylstannyl)vinyl amino acids, respectively, in three steps {(i) alkylative side chain installation, (ii) eliminative ring-opening and (iii) vinyl selenide to vinyl stannane interconversion}. The title compounds may be protodestannylated to the corresponding free, quaternary L- and D-vinyl amino acids. Alternatively, the 2-stannylvinyl α-branch (or the derivative 2-iodovinyl branch) may be exploited to access novel quaternary, L- and D-β,γ-unsaturated amino acids via a range of transition metal-mediated cross coupling reactions.
Keywords: chain extension; self regeneration of stereocenters (SRS); vinyl selenides; vinyl stannanes; β; γ-unsaturated amino acids.
Figures
References
-
- Williams RM. Synthesis of Optically Active Amino Acids. Pergamon Press; Oxford: 1989.
-
- Dardenne G, Casimir J, Marlier M, Larsen PO. Phytochemistry. 1974;13:1897–1900.
-
- Cho C, Ishii R, Hyeon S, Suzuki A. Agric Biol Chem. 1987;51:2597–2598.
- Cornell NW, Zuurendonk PF, Kerich MJ, Straight CB. Biochem J. 1984;220:707–716. - PMC - PubMed
- Soper TS, Manning JM, Marcotte PA, Walsh CT. J Biol Chem. 1977;252:1571–1575. - PubMed
- Rando RR, Relyea N, Cheng L. J Biol Chem. 1976;251:3306–3312. - PubMed
- Rando RR. Biochemistry. 1974;13:3859–3863. - PubMed
-
- Griffith OW. J Biol Chem. 1983;258:1591–1598. - PubMed
-
- Zabriskie TM, Cheng H, Vederas JC. J Am Chem Soc. 1992;114:2270–2272.
Grants and funding
LinkOut - more resources
Full Text Sources