Practical, efficient, and broadly applicable synthesis of readily differentiable vicinal diboronate compounds by catalytic three-component reactions
- PMID: 29973744
- PMCID: PMC6027584
- DOI: 10.1016/j.tet.2017.05.068
Practical, efficient, and broadly applicable synthesis of readily differentiable vicinal diboronate compounds by catalytic three-component reactions
Abstract
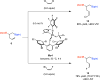
A practical, efficient and broadly applicable catalytic method for synthesis of easily differentiable vicinal diboronate compounds is presented. Reactions are promoted by a combination of PCy3 or PPh3, CuCl and LiOt-Bu and may be performed with readily accessible alkenyl boronate substrates. Through the use of an alkenyl-B(pin) (pin = pinacolato) or alkenyl- B(dan) (dan = naphthalene-1,8-diaminato) starting material and commercially available (pin)B- B(dan) or B2(pin)2 as the reagent, a range of vicinal diboronates, including those that contain a B-substituted quaternary carbon center, may be prepared in up to 91% yield and with >98% site selectivity. High enantioselectivities can be obtained (up to 96:4 er) through the use of commercially available chiral bis-phosphine ligands for reactions that afford mixed diboronate products.
Keywords: Allylic substitution; Boron; Catalysis; Copper; Enantioselective synthesis; Vicinal diboron compounds.
Figures




References
-
-
For representative reports, see:
- Trudeau S, Morgan JB, Shreshta M, Morken JP. J Org Chem. 2005;70:9538–9544. - PubMed
- Coombs JR, Haeffner F, Kliman LT, Morken JP. J Am Chem Soc. 2013;135:11222–11231. - PMC - PubMed
-
For processes promoted by Rh-based complexes, see:
- Toribatake K, Nishiyama H. Angew Chem Int Ed. 2013;52:11011–11015. - PubMed
-
-
- Bonet A, Pubill-Ulldemolins C, Bo C, Gulyás H, Fernandez E. Angew Chem Int Ed. 2011;50:7158–7161. - PubMed
- Blaisdell TP, Caya TC, Zhang L, Sanz-Marco A, Morken JP. J Am Chem Soc. 2014;136:9264–9267. - PMC - PubMed
- Miralles N, Cid J, Cuenca AB, Carbó JJ, Fernandez E. Chem Commun. 2015;51:1693–1696. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
