Estrogen Protects Neurotransmission Transcriptome During Status Epilepticus
- PMID: 29973860
- PMCID: PMC6019481
- DOI: 10.3389/fnins.2018.00332
Estrogen Protects Neurotransmission Transcriptome During Status Epilepticus
Abstract
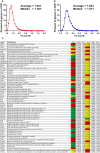
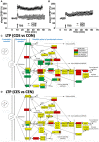
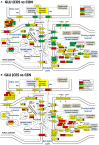
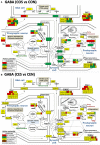
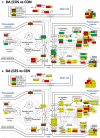
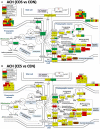
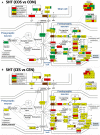
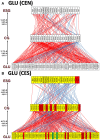
Women with epilepsy commonly have premature onset of menopause. The decrease in estrogen levels is associated with increased occurrence of neurodegenerative processes and cognitive decline. Previously, we found that estradiol (E2) replacement in ovariectomized (OVX) female rats significantly reduced the seizure-related damage in the sensitive hilar region of hippocampal dentate gyrus (DG). However, the complex mechanisms by which E2 empowers the genomic fabrics of neurotransmission to resist damaging effects of status epilepticus (SE) are still unclear. We determined the protective effects of the estradiol replacement against kainic acid-induced SE-associated transcriptomic alterations in the DG of OVX rats. Without E2 replacement, SE altered expression of 44% of the DG genes. SE affected all major functional pathways, including apoptosis (61%), Alzheimer's disease (47%), cell cycle (59%), long-term potentiation (62%), and depression (55%), as well as synaptic vesicle cycle (62%), glutamatergic (53%), GABAergic (49%), cholinergic (52%), dopaminergic (55%), and serotonergic (49%) neurotransmission. However, in rats with E2 replacement the percentage of significantly affected genes after SE was reduced to the average 11% (from 8% for apoptosis to 32% for GABAergic synapse). Interestingly, while SE down-regulated most of the synaptic receptor genes in oil-injected females it had little effect on these receptors after E2-replacement. Our novel Pathway Protection analysis indicated that the E2-replacement prevented SE-related damage from 50% for GABA to 75% for dopaminergic transmission. The 15% synergistic expression between genes involved in estrogen signaling (ESG) and neurotransmission explains why low E2 levels result in down-regulation of neurotransmission. Interestingly, in animals with E2-replacement, SE switched 131 synergistically expressed ESG-neurotransmission gene pairs into antagonistically expressed gene pairs. Thus, the ESG pathway acts like a buffer against SE-induced alteration of neurotransmission that may contribute to the E2-mediated maintenance of brain function after the SE injury in postmenopausal women. We also show that the long-term potentiation is lost in OVX rats following SE but not in those with E2 replacement. The electrophysiological findings in OVX female rats with SE are corroborated by the high percentage of long-term potentiation regulated genes (62%) in oil-injected while only 13% of genes were regulated following SE in E2-replaced rats.
Keywords: GABAergic synapse; beta-estradiol; cholinergic synapse; dentate gyrus; dopaminergic synapse; glutamatergic synapse; jun oncogene; serotonergic synapse.
Figures





Similar articles
-
Estrogen treatment protects GABA(B) inhibition in the dentate gyrus of female rats after kainic acid-induced status epilepticus.Epilepsia. 2002;43 Suppl 5:146-51. doi: 10.1046/j.1528-1157.43.s.5.3.x. Epilepsia. 2002. PMID: 12121310
-
Estradiol replacement alters expression of genes related to neurotransmission and immune surveillance in the frontal cortex of middle-aged, ovariectomized rats.Endocrinology. 2010 Aug;151(8):3847-62. doi: 10.1210/en.2010-0375. Epub 2010 Jun 9. Endocrinology. 2010. PMID: 20534718
-
The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats.Endocrinology. 1995 May;136(5):2320-4. doi: 10.1210/endo.136.5.7720680. Endocrinology. 1995. PMID: 7720680
-
Role of estrogen replacement therapy in memory enhancement and the prevention of neuronal loss associated with Alzheimer's disease.Am J Med. 1997 Sep 22;103(3A):19S-25S. doi: 10.1016/s0002-9343(97)00260-x. Am J Med. 1997. PMID: 9344403 Review.
-
Chronic epileptogenic cellular alterations in the limbic system after status epilepticus.Epilepsia. 1999;40 Suppl 1:S23-33; discussion S40-1. doi: 10.1111/j.1528-1157.1999.tb00875.x. Epilepsia. 1999. PMID: 10421558 Review.
Cited by
-
Powerful quantifiers for cancer transcriptomics.World J Clin Oncol. 2020 Sep 24;11(9):679-704. doi: 10.5306/wjco.v11.i9.679. World J Clin Oncol. 2020. PMID: 33033692 Free PMC article. Review.
-
Regeneration of neurotransmission transcriptome in a model of epileptic encephalopathy after antiinflammatory treatment.Neural Regen Res. 2018 Oct;13(10):1715-1718. doi: 10.4103/1673-5374.238607. Neural Regen Res. 2018. PMID: 30136682 Free PMC article. Review.
-
Atrazine exposure in zebrafish induces aberrant genome-wide methylation.Neurotoxicol Teratol. 2022 Jul-Aug;92:107091. doi: 10.1016/j.ntt.2022.107091. Epub 2022 Apr 23. Neurotoxicol Teratol. 2022. PMID: 35472415 Free PMC article.
-
Coordinated Activity of Transcriptional Networks Responding to the Pattern of Action Potential Firing in Neurons.Genes (Basel). 2019 Sep 26;10(10):754. doi: 10.3390/genes10100754. Genes (Basel). 2019. PMID: 31561430 Free PMC article.
-
Activation of metabotropic glutamate receptor 1 regulates hippocampal CA1 region excitability in rats with status epilepticus by suppressing the HCN1 channel.Neural Regen Res. 2023 Mar;18(3):594-602. doi: 10.4103/1673-5374.350206. Neural Regen Res. 2023. PMID: 36018183 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

