Imbalanced Expression of Tau and Tubulin Induces Neuronal Dysfunction in C. elegans Models of Tauopathy
- PMID: 29973863
- PMCID: PMC6019497
- DOI: 10.3389/fnins.2018.00415
Imbalanced Expression of Tau and Tubulin Induces Neuronal Dysfunction in C. elegans Models of Tauopathy
Abstract
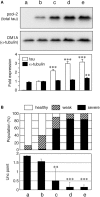
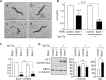
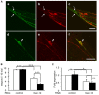
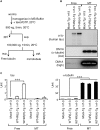
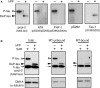
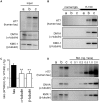
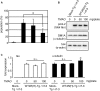
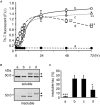
Tauopathy is a type of dementia defined by the accumulation of filamentous tau inclusions in neural cells. Most types of dementia in the elderly, including Alzheimer's disease, are tauopathies. Although it is believed that tau protein abnormalities and/or the loss of its functions results in neurodegeneration and dementia, the mechanism of tauopathy remains obscure. Loss of microtubules and/or tubulin is a known consequence of tau accumulating in neurons in Alzheimer's disease. In other words, there is an excess level of tau relative to tubulin in tauopathy neurons. To test whether this imbalance of tau and tubulin expression results in the neurotoxicity of tau, we developed several transgenic C. elegans lines that express human tau at various levels in pan-neurons. These worms showed behavioral abnormalities in a tau expression-dependent manner. The knockdown of a tubulin-specific chaperon, or a subset of tubulin, led to enhanced tau toxicity even in low-expressing tau-transgenic worms that showed no abnormal behaviors. In addition, the suppression of tau expression in tubulin knockdown worms rescued neuronal dysfunction. Thus, not only the overexpression of tau but also a reduction in tubulin can trigger the neurotoxicity of tau. Tau expressed in worms was also highly phosphorylated and largely bound to tubulin dimers rather than microtubules. Relative amount of tubulin-unbound tau was increased in high-expressing tau-transgenic worms showing tau toxicity. We further demonstrated that tau aggregation was inhibited by co-incubation of purified tubulin in vitro, meaning sufficient amounts of tubulin can protect against the formation of tau inclusions. These results suggest that the expression ratio of tau to tubulin may be a determinant of the tauopathy cascade.
Keywords: Alzheimer’s disease; C. elegans; microtubule; neurodegeneration; tau; tauopathy; tubulin.
Figures

Similar articles
-
Inhibition of microtubule assembly competent tubulin synthesis leads to accumulation of phosphorylated tau in neuronal cell bodies.Biochem Biophys Res Commun. 2020 Jan 15;521(3):779-785. doi: 10.1016/j.bbrc.2019.10.191. Epub 2019 Nov 5. Biochem Biophys Res Commun. 2020. PMID: 31699369
-
Distinct Poly(A) nucleases have differential impact on sut-2 dependent tauopathy phenotypes.Neurobiol Dis. 2021 Jan;147:105148. doi: 10.1016/j.nbd.2020.105148. Epub 2020 Oct 25. Neurobiol Dis. 2021. PMID: 33184027 Free PMC article.
-
Curcumin improves tau-induced neuronal dysfunction of nematodes.Neurobiol Aging. 2016 Mar;39:69-81. doi: 10.1016/j.neurobiolaging.2015.11.004. Epub 2015 Dec 1. Neurobiol Aging. 2016. PMID: 26923403
-
Positron Emission Tomography in Animal Models of Tauopathies.Front Aging Neurosci. 2022 Jan 10;13:761913. doi: 10.3389/fnagi.2021.761913. eCollection 2021. Front Aging Neurosci. 2022. PMID: 35082657 Free PMC article. Review.
-
Regulation of neuronal microtubule dynamics by tau: Implications for tauopathies.Int J Biol Macromol. 2019 Jul 15;133:473-483. doi: 10.1016/j.ijbiomac.2019.04.120. Epub 2019 Apr 17. Int J Biol Macromol. 2019. PMID: 31004638 Review.
Cited by
-
Neuroprotective Potential of the Flavonoids Quercetin and Epicatechin in a C. elegans Tauopathy Model.Mol Nutr Food Res. 2025 Aug;69(15):e70108. doi: 10.1002/mnfr.70108. Epub 2025 May 12. Mol Nutr Food Res. 2025. PMID: 40351085 Free PMC article.
-
Comparative functional genomic analysis of Alzheimer's affected and naturally aging brains.PeerJ. 2020 Mar 20;8:e8682. doi: 10.7717/peerj.8682. eCollection 2020. PeerJ. 2020. PMID: 32219020 Free PMC article.
-
The role of wild-type tau in Alzheimer's disease and related tauopathies.J Life Sci (Westlake Village). 2020 Dec;2(4):1-17. doi: 10.36069/jols/20201201. J Life Sci (Westlake Village). 2020. PMID: 33665646 Free PMC article.
-
Selective disruption of Drp1-independent mitophagy and mitolysosome trafficking by an Alzheimer's disease relevant tau modification in a novel Caenorhabditis elegans model.Genetics. 2022 Aug 30;222(1):iyac104. doi: 10.1093/genetics/iyac104. Genetics. 2022. PMID: 35916724 Free PMC article.
-
The role of protein complexes in human genetic disease.Protein Sci. 2019 Aug;28(8):1400-1411. doi: 10.1002/pro.3667. Epub 2019 Jul 1. Protein Sci. 2019. PMID: 31219644 Free PMC article. Review.
References
-
- Alonso A. D., Grundke-Iqbal I., Barra H. S., Iqbal K. (1997). Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc. Natl. Acad. Sci. U.S.A. 94 298–303. 10.1073/pnas.94.1.298 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

