Endocannabinoid Signaling at Hypothalamic Steroidogenic Factor-1/Proopiomelanocortin Synapses Is Sex- and Diet-Sensitive
- PMID: 29973869
- PMCID: PMC6020785
- DOI: 10.3389/fnmol.2018.00214
Endocannabinoid Signaling at Hypothalamic Steroidogenic Factor-1/Proopiomelanocortin Synapses Is Sex- and Diet-Sensitive
Abstract
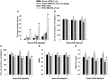
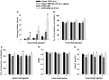
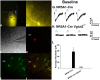
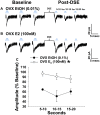
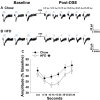
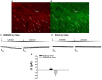
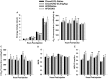
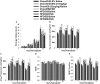
We tested the hypotheses that steroidogenic factor (SF)-1 neurons in the hypothalamic ventromedial nucleus (VMN) provide sexually disparate, endocannabinoid (EC)- and diet-sensitive glutamatergic input onto proopiomelanocortin (POMC) neurons. Electrophysiological recordings were performed in hypothalamic slices from intact and castrated guinea pigs, along with in vitro optogenetic experiments in intact male as well as cycling and ovariectomized female NR5A1-Cre mice. In slices from castrated male and female guinea pigs, depolarized-induced suppression of excitation (DSE) time-dependently reduced the amplitude of evoked excitatory postsynaptic currents (eEPSCs) in POMC neurons generated by electrically stimulating the dorsomedial VMN. Androgen stimulation rapidly enhanced this DSE, which was also found in insulin-resistant, high-fat diet (HFD)-fed males. By contrast, retrograde signaling at VMN/ARC POMC synapses was markedly attenuated in periovulatory females. HFD potentiated central cannabinoid-induced hyperphagia in both males and females, but exerted differential influences on cannabinoid-induced increases in energy expenditure. In NR5A1-Cre mice, the reduction in light-evoked EPSC amplitude caused by postsynaptic depolarization in cycling females was modest in comparison to that seen in intact males. Estradiol attenuated the DSE in light-evoked EPSC amplitude in slices from ovariectomized females. Moreover, the retrograde inhibition of transmission was further accentuated in HFD-fed males. Chemogenetic activation of SF-1 neurons suppressed appetite and increased energy expenditure in males, effects which were attenuated by HFD. Conversely, energy expenditure was increased in estradiol- but not vehicle-treated ovariectomized females. Together with our previous studies indicating that DSE in POMC neurons is EC-mediated, these findings indicate that VMN SF-1/ARC POMC synapses represent a sexually differentiated, EC- and diet-sensitive anorexigenic component within the hypothalamic energy balance circuitry.
Keywords: endocannabinoids; estradiol; insulin; obesity; proopiomelanocortin; sex differences; steroidogenic factor-1; testosterone.
Figures




References
-
- Andrews W. W., Advis J. P., Ojeda S. R. (1981). The maturation of estradiol-negative feedback in female rats: evidence that the resetting of the hypothalamic “gonadostat” does not precede the first preovulatory surge of gonadotropins. Endocrinology 109, 2022–2031. 10.1210/endo-109-6-2022 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

