Pre-α-pro-GDNF and Pre-β-pro-GDNF Isoforms Are Neuroprotective in the 6-hydroxydopamine Rat Model of Parkinson's Disease
- PMID: 29973907
- PMCID: PMC6019446
- DOI: 10.3389/fneur.2018.00457
Pre-α-pro-GDNF and Pre-β-pro-GDNF Isoforms Are Neuroprotective in the 6-hydroxydopamine Rat Model of Parkinson's Disease
Abstract
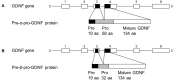
Glial cell line-derived neurotrophic factor (GDNF) is one of the most studied neurotrophic factors. GDNF has two splice isoforms, full-length pre-α-pro-GDNF (α-GDNF) and pre-β-pro-GDNF (β-GDNF), which has a 26 amino acid deletion in the pro-region. Thus far, studies have focused solely on the α-GDNF isoform, and nothing is known about the in vivo effects of the shorter β-GDNF variant. Here we compare for the first time the effects of overexpressed α-GDNF and β-GDNF in non-lesioned rat striatum and the partial 6-hydroxydopamine lesion model of Parkinson's disease. GDNF isoforms were overexpressed with their native pre-pro-sequences in the striatum using an adeno-associated virus (AAV) vector, and the effects on motor performance and dopaminergic phenotype of the nigrostriatal pathway were assessed. In the non-lesioned striatum, both isoforms increased the density of dopamine transporter-positive fibers at 3 weeks after viral vector delivery. Although both isoforms increased the activity of the animals in cylinder assay, only α-GDNF enhanced the use of contralateral paw. Four weeks later, the striatal tyrosine hydroxylase (TH)-immunoreactivity was decreased in both α-GDNF and β-GDNF treated animals. In the neuroprotection assay, both GDNF splice isoforms increased the number of TH-immunoreactive cells in the substantia nigra but did not promote behavioral recovery based on amphetamine-induced rotation or cylinder assays. Thus, the shorter GDNF isoform, β-GDNF, and the full-length α-isoform have comparable neuroprotective efficacy on dopamine neurons of the nigrostriatal circuitry.
Keywords: GDNF; alternative splicing; dopamine; neurodegeneration; neurotrophic factors; splice variant; tyrosine hydroxylase.
Figures



References
-
- Sauer H, Rosenblad C, Bjorklund A. Glial cell line-derived neurotrophic factor but not transforming growth factor beta 3 prevents delayed degeneration of nigral dopaminergic neurons following striatal 6-hydroxydopamine lesion. Proc Natl Acad Sci USA. (1995) 92:8935–9. 10.1073/pnas.92.19.8935 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

