Equal Expansion of Endogenous Transplant-Specific Regulatory T Cell and Recruitment Into the Allograft During Rejection and Tolerance
- PMID: 29973932
- PMCID: PMC6020780
- DOI: 10.3389/fimmu.2018.01385
Equal Expansion of Endogenous Transplant-Specific Regulatory T Cell and Recruitment Into the Allograft During Rejection and Tolerance
Abstract
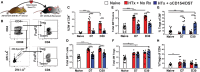
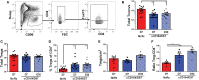
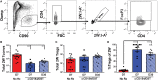
Despite numerous advances in the definition of a role for regulatory T cells (Tregs) in facilitating experimental transplantation tolerance, and ongoing clinical trials for Treg-based therapies, critical issues related to the optimum dosage, antigen-specificity, and Treg-friendly adjunct immunosuppressants remain incompletely resolved. In this study, we used a tractable approach of MHC tetramers and flow cytometry to define the fate of conventional (Tconvs) and Tregs CD4+ T cells that recognize donor 2W antigens presented by I-Ab on donor and recipient antigen-presenting cells (APCs) in a mouse cardiac allograft transplant model. Our study shows that these endogenous, donor-reactive Tregs comparably accumulate in the spleens of recipients undergoing acute rejection or exhibiting costimulation blockade-induced tolerance. Importantly, this expansion was not detected when analyzing bulk splenic Tregs. Systemically, the distinguishing feature between tolerance and rejection was the inhibition of donor-reactive conventional T cell (Tconv) expansion in tolerance, translating into increased percentages of splenic FoxP3+ Tregs within the 2W:I-Ab CD4+ T cell subset compared to rejection (~35 vs. <5% in tolerance vs. rejection). We further observed that continuous administration of rapamycin, cyclosporine A, or CTLA4-Ig did not facilitate donor-specific Treg expansion, while all three drugs inhibited Tconv expansion. Finally, donor-specific Tregs accumulated comparably in rejecting tolerant allografts, whereas tolerant grafts harbored <10% of the donor-specific Tconv numbers observed in rejecting allografts. Thus, ~80% of 2W:I-Ab CD4+ T cells in tolerant allografts expressed FoxP3+ compared to ≤10% in rejecting allografts. A similar, albeit lesser, enrichment was observed with bulk graft-infiltrating CD4+ cells, where ~30% were FoxP3+ in tolerant allografts, compared to ≤10% in rejecting allografts. Finally, we assessed that the phenotype of 2W:I-Ab Tregs and observed that the percentages of cells expressing neuropilin-1 and CD73 were significantly higher in tolerance compared to rejection, suggesting that these Tregs may be functionally distinct. Collectively, the analysis of donor-reactive, but not of bulk, Tconvs and Tregs reveal a systemic signature of tolerance that is stable and congruent with the signature within tolerant allografts. Our data also underscore the importance of limiting Tconv expansion for high donor-specific Tregs:Tconv ratios to be successfully attained in transplantation tolerance.
Keywords: allospecific T cells; conventional T cells; costimulatory blockade; immunosuppression; murine heart transplant; regulatory T cells; transplant tolerance; transplantation immunology.
Figures


Similar articles
-
CAR Treg synergy with anti-CD154 promotes infectious tolerance and dictates allogeneic heart transplant acceptance.JCI Insight. 2025 Apr 8;10(7):e188624. doi: 10.1172/jci.insight.188624. JCI Insight. 2025. PMID: 40197364 Free PMC article.
-
The regulatory T cell effector molecule fibrinogen-like protein 2 is necessary for the development of rapamycin-induced tolerance to fully MHC-mismatched murine cardiac allografts.Immunology. 2015 Jan;144(1):91-106. doi: 10.1111/imm.12354. Immunology. 2015. PMID: 24990517 Free PMC article.
-
Induction of Foxp3-expressing regulatory T-cells by donor blood transfusion is required for tolerance to rat liver allografts.PLoS One. 2009 Nov 23;4(11):e7840. doi: 10.1371/journal.pone.0007840. PLoS One. 2009. PMID: 19956764 Free PMC article.
-
Alloantigen specific T regulatory cells in transplant tolerance.Int Immunopharmacol. 2009 May;9(5):570-4. doi: 10.1016/j.intimp.2009.01.016. Epub 2009 Jan 29. Int Immunopharmacol. 2009. PMID: 19539571 Review.
-
The Limits of Linked Suppression for Regulatory T Cells.Front Immunol. 2016 Mar 9;7:82. doi: 10.3389/fimmu.2016.00082. eCollection 2016. Front Immunol. 2016. PMID: 27014262 Free PMC article. Review.
Cited by
-
CAR Treg synergy with anti-CD154 promotes infectious tolerance and dictates allogeneic heart transplant acceptance.JCI Insight. 2025 Apr 8;10(7):e188624. doi: 10.1172/jci.insight.188624. JCI Insight. 2025. PMID: 40197364 Free PMC article.
-
Outstanding questions in transplantation: B cells, alloantibodies, and humoral rejection.Am J Transplant. 2019 Aug;19(8):2155-2163. doi: 10.1111/ajt.15323. Epub 2019 Apr 10. Am J Transplant. 2019. PMID: 30803121 Free PMC article. Review.
-
Classic and Current Opinions in Human Organ and Tissue Transplantation.Cureus. 2022 Nov 1;14(11):e30982. doi: 10.7759/cureus.30982. eCollection 2022 Nov. Cureus. 2022. PMID: 36337306 Free PMC article. Review.
-
Mesenchymal stem cells augment regulatory T cell function via CD80-mediated interactions and promote allograft survival.Am J Transplant. 2022 Jun;22(6):1564-1577. doi: 10.1111/ajt.17001. Epub 2022 Feb 26. Am J Transplant. 2022. PMID: 35170213 Free PMC article.
-
Tregs in transplantation tolerance: role and therapeutic potential.Front Transplant. 2023 Aug 30;2:1217065. doi: 10.3389/frtra.2023.1217065. eCollection 2023. Front Transplant. 2023. PMID: 38993904 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

