Evoked and Ongoing Pain-Like Behaviours in a Rat Model of Paclitaxel-Induced Peripheral Neuropathy
- PMID: 29973969
- PMCID: PMC6008701
- DOI: 10.1155/2018/8217613
Evoked and Ongoing Pain-Like Behaviours in a Rat Model of Paclitaxel-Induced Peripheral Neuropathy
Abstract
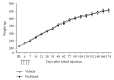
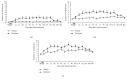
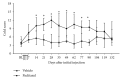
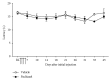
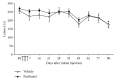
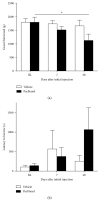
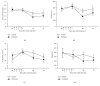
Paclitaxel-induced neuropathic pain is a major dose-limiting side effect of paclitaxel therapy. This study characterises a variety of rat behavioural responses induced by intermittent administration of clinically formulated paclitaxel. 2 mg/kg paclitaxel or equivalent vehicle was administered intraperitoneally on days 0, 2, 4, and 6 to adult male Sprague-Dawley rats. Evoked pain-like behaviours were assessed with von Frey filaments, acetone, or radiant heat application to plantar hind paws to ascertain mechanical, cold, or heat sensitivity, respectively. Motor coordination was evaluated using an accelerating RotaRod apparatus. Ongoing pain-like behaviour was assessed via spontaneous burrowing and nocturnal wheel running. Mechanical and cold hypersensitivity developed after a delayed onset, peaked approximately on day 28, and persisted for several months. Heat sensitivity and motor coordination were unaltered in paclitaxel-treated rats. Spontaneous burrowing behaviour and nocturnal wheel running were significantly impaired on day 28, but not on day 7, indicating ongoing pain-like behaviour, rather than acute drug toxicity. This study comprehensively characterises a rat model of paclitaxel-induced peripheral neuropathy, providing the first evidence for ongoing pain-like behaviour, which occurs in parallel with maximal mechanical/cold hypersensitivity. We hope that this new data improve the face validity of rat models to better reflect patient-reported pain symptoms, aiding translation of new treatments to the clinic.
Figures
References
-
- Augusto C., Pietro M., Cinzia M., et al. Peripheral neuropathy due to paclitaxel: study of the temporal relationships between the therapeutic schedule and the clinical quantitative score (QST) and comparison with neurophysiological findings. Journal of Neuro-Oncology. 2008;86(1):89–99. doi: 10.1007/s11060-007-9438-8. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

