All-Inorganic Metal Halide Perovskite Nanocrystals: Opportunities and Challenges
- PMID: 29974062
- PMCID: PMC6026778
- DOI: 10.1021/acscentsci.8b00201
All-Inorganic Metal Halide Perovskite Nanocrystals: Opportunities and Challenges
Abstract
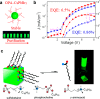
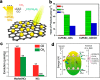
The past decade has witnessed the growing interest in metal halide perovskites as driven by their promising applications in diverse fields. The low intrinsic stability of the early developed organic versions has however hampered their widespread applications. Very recently, all-inorganic perovskite nanocrystals have emerged as a new class of materials that hold great promise for the practical applications in solar cells, photodetectors, light-emitting diodes, and lasers, among others. In this Outlook, we first discuss the recent developments in the preparation, properties, and applications of all-inorganic metal halide perovskite nanocrystals, with a particular focus on CsPbX3, and then provide our view of current challenges and future directions in this emerging area. Our goal is to introduce the current status of this type of new materials to researchers from different areas and motivate them to explore all the potentials.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Yoshikawa K.; Kawasaki H.; Yoshida W.; Irie T.; Konishi K.; Nakano K.; Uto T.; Adachi D.; Kanematsu M.; Uzu H.; Yamamoto K. Silicon heterojunction solar cell with interdigitated back contacts for a photoconversion efficiency over 26%. Nat. Energy 2017, 2, 17032. 10.1038/nenergy.2017.32. - DOI
-
- Christians J. A.; Schulz P.; Tinkham J. S.; Schloemer T. H.; Harvey S. P.; Tremolet de Villers B. J.; Sellinger A.; Berry J. J.; Luther J. M. Tailored interfaces of unencapsulated perovskite solar cells for > 1,000 h operational stability. Nat. Energy 2018, 3, 68–74. 10.1038/s41560-017-0067-y. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

