Multicenter phase 1/2 study of forodesine in patients with relapsed peripheral T cell lymphoma
- PMID: 29974231
- PMCID: PMC6334730
- DOI: 10.1007/s00277-018-3418-2
Multicenter phase 1/2 study of forodesine in patients with relapsed peripheral T cell lymphoma
Erratum in
-
Correction to: Multicenter phase 1/2 study of forodesine in patients with relapsed peripheral T cell lymphoma.Ann Hematol. 2018 Dec;97(12):2529-2530. doi: 10.1007/s00277-018-3455-x. Ann Hematol. 2018. PMID: 30054705 Free PMC article.
Abstract
Peripheral T cell lymphomas are an aggressive group of non-Hodgkin lymphomas with poor outcomes for most subtypes and no accepted standard of care for relapsed patients. This study evaluated the efficacy and safety of forodesine, a novel purine nucleoside phosphorylase inhibitor, in patients with relapsed peripheral T cell lymphomas. Patients with histologically confirmed disease, progression after ≥ 1 prior treatment, and an objective response to last treatment received oral forodesine 300 mg twice-daily. The primary endpoint was objective response rate (ORR). Secondary endpoints included duration of response, progression-free survival (PFS), overall survival (OS), and safety. Forty-eight patients (median age, 69.5 years; median of 2 prior treatments) received forodesine. In phase 1 (n = 3 evaluable), no dose-limiting toxicity was observed during the first 28 days of forodesine treatment. In phase 2 (n = 41 evaluable), the ORR for the primary and final analyses was 22% (90% CI 12-35%) and 25% (90% CI 14-38%), respectively, including four complete responses (10%). Median PFS and OS were 1.9 and 15.6 months, respectively. The most common grade 3/4 adverse events were lymphopenia (96%), leukopenia (42%), and neutropenia (35%). Dose reduction and discontinuation due to adverse events were uncommon. Secondary B cell lymphoma developed in five patients, of whom four were positive for Epstein-Barr virus. In conclusion, forodesine has single-agent activity within the range of approved therapies in relapsed peripheral T cell lymphomas, with a manageable safety profile, and may represent a viable treatment option for this difficult-to-treat population.
Keywords: Forodesine; Overall response rate; Peripheral T cell lymphoma; Progression-free survival; Purine nucleoside phosphorylase inhibitor.
Conflict of interest statement
Conflict of interest
Dai Maruyama reports research funding from Mundipharma K.K., Chugai Pharma, Kyowa Hakko Kirin, Ono Pharmaceutical, Celgene, Janssen, GlaxoSmithKline, Eisai, Takeda, SERVIER, and Abbvie, MSD, and receiving honoraria from Mundipharma K.K., Takeda, Janssen, Eisai, Biomedis International, Celgene, Sanofi, Kyowa Hakko Kirin, Fujifilm, Mochida Pharmaceutical Co., Ltd., Ono Pharmaceutical, and Chugai Pharma. Toshiki Uchida reports receiving an honorarium from Janssen. Yoshinobu Maeda reports research funding from Chugai Pharma, Kyowa Hakko Kirin, and Ono Pharmaceutical, and an honorarium from Mundipharma K.K. Hirokazu Nagai reports research funding from Chugai Pharma, Mundipharma K.K., Eisai, Sanofi K.K., Janssen, Takeda, Ono Pharmaceutical, and Kyowa Hakko Kirin. Hirohiko Shibayama reports research funding from Takeda, Janssen, Ono Pharmaceutical, Astellas, Teijin Pharma, Shionogi, Taiho, and Mundipharma KK, and honoraria from Takeda, Celgene, Novartis, Janssen, Kyowa Hakko Kirin, and Mundipharma KK. Kiyoshi Ando reports research funding from Mundipharma K.K. Isao Yoshida reports research funding from Kyowa Hakko Kirin and Chugai Pharma, and receiving honoraria from Mundipharma K.K., Celgene, and Kyowa Hakko Kirin. Michihiro Hidaka reports research funding from Mundipharma K.K. and Chugai Pharma. Tohru Murayama reports research funding from Celltrion, and honoraria from Nippon Shinyaku, Taiho Pharma, Janssen, Siemens, Kyowa Hakko Kirin, Novartis, Celgene, Ono Pharmaceutical, Pfizer, and Bristol-Myers Squibb. Junji Suzumiya reports research funding from Kyowa Hakko Kirin, Chugai-Roche, Shinnipponkagaku-PPD, Astellas, Toyama Chemical, Eisai, Takeda, Sumitomo Dainippon, Shionogi, Taiho, Yakult, and SymBio, and receiving honoraria from Kyowa Hakko Kirin, Chugai-Roche, Janssen, Eisai, Takeda, Sumitomo Dainippon, Otsuka, Celgene, Alexion, Novartis, Pfizer, AstraZeneca, Bristol-Myers Squibb, Merck, Ono Pharmaceutical, Eli Lilly, Shire, Gilead, and Zenyaku. Kazuo Tamura reports receiving honoraria from Ono Pharmaceutical, Kyowa Hakko Kirin, and Eli Lilly. Ryuzo Ueda reports research funding and an honorarium from Kyowa Hakko Kirin, serving as a consultant for Mundipharma K.K. and Terumo, and receiving an honorarium from Chugai Pharma. Kensei Tobinai reports receiving research funding from Mundipharma K.K., Chugai Pharma, Kyowa Hakko Kirin, Ono Pharmaceutical, Celgene, Janssen, GlaxoSmithKline, Eisai, Takeda, Servier, and Abbvie, and honoraria from Zenyaku Kogyo, Eisai, Takeda, Mundipharma K.K., Janssen, HUYA Bioscience International, Kyowa Hakko Kirin, Celgene, Chugai Pharma, and Ono Pharmaceutical. All remaining authors declared that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declarations and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Figures
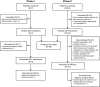
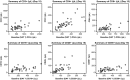

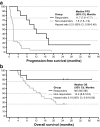
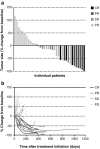

Similar articles
-
Phase I study of BCX1777 (forodesine) in patients with relapsed or refractory peripheral T/natural killer-cell malignancies.Cancer Sci. 2012 Jul;103(7):1290-5. doi: 10.1111/j.1349-7006.2012.02287.x. Epub 2012 Apr 30. Cancer Sci. 2012. PMID: 22448814 Free PMC article. Clinical Trial.
-
Final results of a multicenter phase II study of the purine nucleoside phosphorylase (PNP) inhibitor forodesine in patients with advanced cutaneous T-cell lymphomas (CTCL) (Mycosis fungoides and Sézary syndrome).Ann Oncol. 2014 Sep;25(9):1807-1812. doi: 10.1093/annonc/mdu231. Epub 2014 Jun 19. Ann Oncol. 2014. PMID: 24948692 Clinical Trial.
-
Forodesine in the treatment of cutaneous T-cell lymphoma.Expert Opin Investig Drugs. 2017 Jun;26(6):771-775. doi: 10.1080/13543784.2017.1324569. Epub 2017 May 5. Expert Opin Investig Drugs. 2017. PMID: 28447489 Review.
-
Forodesine in the treatment of relapsed/refractory peripheral T-cell lymphoma: an evidence-based review.Onco Targets Ther. 2018 Apr 20;11:2287-2293. doi: 10.2147/OTT.S140756. eCollection 2018. Onco Targets Ther. 2018. PMID: 29719411 Free PMC article. Review.
-
Phase 2 and pharmacodynamic study of oral forodesine in patients with advanced, fludarabine-treated chronic lymphocytic leukemia.Blood. 2010 Aug 12;116(6):886-92. doi: 10.1182/blood-2010-02-272039. Epub 2010 Apr 28. Blood. 2010. PMID: 20427701 Free PMC article. Clinical Trial.
Cited by
-
Past, present and future therapeutic approaches in nodal peripheral T-cell lymphomas.Haematologica. 2023 Dec 1;108(12):3211-3226. doi: 10.3324/haematol.2021.280275. Haematologica. 2023. PMID: 38037799 Free PMC article.
-
Novel clinical risk stratification and treatment strategies in relapsed/refractory peripheral T-cell lymphoma.J Hematol Oncol. 2024 Jun 1;17(1):38. doi: 10.1186/s13045-024-01560-7. J Hematol Oncol. 2024. PMID: 38824603 Free PMC article. Review.
-
Purine nucleoside phosphorylase enables dual metabolic checkpoints that prevent T cell immunodeficiency and TLR7-associated autoimmunity.J Clin Invest. 2022 Aug 15;132(16):e160852. doi: 10.1172/JCI160852. J Clin Invest. 2022. PMID: 35653193 Free PMC article.
-
Landscape of targets within nucleoside metabolism for the modification of immune responses.Front Oncol. 2025 May 30;15:1483769. doi: 10.3389/fonc.2025.1483769. eCollection 2025. Front Oncol. 2025. PMID: 40519286 Free PMC article. Review.
-
Precise diagnosis and targeted therapy of nodal T-follicular helper cell lymphoma (T-FHCL).Front Oncol. 2023 Apr 28;13:1163190. doi: 10.3389/fonc.2023.1163190. eCollection 2023. Front Oncol. 2023. PMID: 37188182 Free PMC article. Review.
References
-
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. Lyon: International Agency for Research on Cancer (IARC); 2008.
-
- NCCN Clinical practice guidelines in oncology. T-cell lymphomas Version 3.2018. http://www.nccn.org. Accessed 13 March 2018
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

