Discriminating radiation injury from recurrent tumor with [18F]PARPi and amino acid PET in mouse models
- PMID: 29974335
- PMCID: PMC6031550
- DOI: 10.1186/s13550-018-0399-z
Discriminating radiation injury from recurrent tumor with [18F]PARPi and amino acid PET in mouse models
Abstract
Background: Radiation injury can be indistinguishable from recurrent tumor on standard imaging. Current protocols for this differential diagnosis require one or more follow-up imaging studies, long dynamic acquisitions, or complex image post-processing; despite much research, the inability to confidently distinguish between these two entities continues to pose a significant dilemma for the treating clinician. Using mouse models of both glioblastoma and radiation necrosis, we tested the potential of poly(ADP-ribose) polymerase (PARP)-targeted PET imaging with [18F]PARPi to better discriminate radiation injury from tumor.
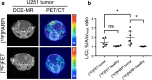
Results: In mice with experimental radiation necrosis, lesion uptake on [18F]PARPi-PET was similar to contralateral uptake (1.02 ± 0.26 lesion/contralateral %IA/ccmax ratio), while [18F]FET-PET clearly delineated the contrast-enhancing region on MR (2.12 ± 0.16 lesion/contralateral %IA/ccmax ratio). In mice with focal intracranial U251 xenografts, tumor visualization on PARPi-PET was superior to FET-PET, and lesion-to-contralateral activity ratios (max/max, p = 0.034) were higher on PARPi-PET than on FET-PET.
Conclusions: A murine model of radiation necrosis does not demonstrate [18F]PARPi avidity, and [18F]PARPi-PET is better than [18F]FET-PET in distinguishing radiation injury from brain tumor. [18F]PARPi-PET can be used for discrimination between recurrent tumor and radiation injury within a single, static imaging session, which may be of value to resolve a common dilemma in neuro-oncology.
Keywords: Amino acid PET; Biomarkers; PARP1; PET/CT; Radiation injury; Radiation necrosis.
Conflict of interest statement
Ethics approval
All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committees of Memorial Sloan Kettering Cancer Center (MSK) or Washington University and followed the National Institutes of Health guidelines for animal welfare.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

