Human peripheral blood mononuclear cells enriched in endothelial progenitor cells via quality and quantity controlled culture accelerate vascularization and wound healing in a porcine wound model
- PMID: 29974793
- PMCID: PMC6158547
- DOI: 10.1177/0963689718780307
Human peripheral blood mononuclear cells enriched in endothelial progenitor cells via quality and quantity controlled culture accelerate vascularization and wound healing in a porcine wound model
Abstract
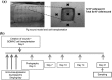
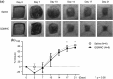
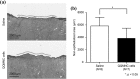
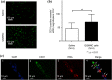
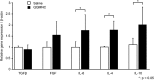
The transplantation of endothelial progenitor cells (EPCs) is used to promote wound angiogenesis. In patients with chronic wounds and accompanying morbidities, EPCs are often compromised in number and function. To overcome these limitations, we previously developed a quality and quantity controlled (QQ) culture system to enrich peripheral blood mononuclear cells (PBMNCs) in EPCs. To evaluate the wound healing efficacy of mononuclear cells (MNCs) harvested after QQ culture (QQMNCs), preclinical studies were performed on large animals. MNCs harvested from the blood of healthy human subjects were cultured in the presence of angiogenic cytokines and growth factors in a serum-free medium for 7 days. A total of 5 × 106 QQMNCs per full-thickness skin defect or control saline was injected into wounds induced in cyclosporine-immunosuppressed pigs. EPC colony-forming assays revealed a significantly higher number of definitive (partially differentiated) EPC colony-forming units in QQMNCs. Flow cytometry evaluation of QQMNC surface markers showed enrichment of CD34+ and CD133+ stem cell populations, significant reduction in CCR2+ cell percentages, and a greater than 10-fold increase in the percentage of anti-inflammatory M2-type macrophages (CD206+ cells) compared with PBMNCs. Wounds treated with QQMNCs had a significantly higher closure rate. Wounds were harvested, frozen, and sectioned at day 21 postoperatively. Hematoxylin and eosin staining revealed that the epithelization of QQMNC-treated wounds was more advanced than in controls. Treated wounds developed granulation tissue with more mature collagen and larger capillary networks. CD31 and human mitochondrial co-staining confirmed the presence of differentiated human cells within newly formed vessels. Real-time polymerase chain reaction (PCR) showed upregulation of interleukin 6 (IL-6), IL-10, and IL-4 in the wound bed, suggesting paracrine activity of the transplanted QQMNCs. Our data demonstrate for the first time that QQ culture of MNCs obtained from a small amount of peripheral blood yields vasculogenic and therapeutic cells effective in wound healing.
Keywords: Chronic wounds; endothelial progenitor cells; peripheral blood mononuclear cells; porcine wound model; vasculogenesis; wound healing.
Conflict of interest statement
Figures


Similar articles
-
Vasculogenic conditioning of peripheral blood mononuclear cells promotes endothelial progenitor cell expansion and phenotype transition of anti-inflammatory macrophage and T lymphocyte to cells with regenerative potential.J Am Heart Assoc. 2014 Jun 25;3(3):e000743. doi: 10.1161/JAHA.113.000743. J Am Heart Assoc. 2014. PMID: 24965023 Free PMC article.
-
Regeneration-associated cells improve recovery from myocardial infarction through enhanced vasculogenesis, anti-inflammation, and cardiomyogenesis.PLoS One. 2018 Nov 28;13(11):e0203244. doi: 10.1371/journal.pone.0203244. eCollection 2018. PLoS One. 2018. PMID: 30485279 Free PMC article.
-
Angiogenic conditioning of peripheral blood mononuclear cells promotes fracture healing.Bone Joint Res. 2017 Aug;6(8):489-498. doi: 10.1302/2046-3758.68.BJR-2016-0338.R1. Bone Joint Res. 2017. PMID: 28835445 Free PMC article.
-
Endothelial progenitor cell therapy for chronic wound tissue regeneration.Cytotherapy. 2019 Nov;21(11):1137-1150. doi: 10.1016/j.jcyt.2019.09.002. Epub 2019 Oct 23. Cytotherapy. 2019. PMID: 31668487 Review.
-
Transplantation of Endothelial Progenitor Cells: Summary and prospect.Acta Histochem. 2023 Jan;125(1):151990. doi: 10.1016/j.acthis.2022.151990. Epub 2022 Dec 30. Acta Histochem. 2023. PMID: 36587456 Review.
Cited by
-
Endothelial Progenitor Cells Conditioned Medium Supports Number of GABAergic Neurons and Exerts Neuroprotection in Cultured Striatal Neuronal Progenitor Cells.Cell Transplant. 2019 Apr;28(4):367-378. doi: 10.1177/0963689719835192. Epub 2019 Apr 24. Cell Transplant. 2019. PMID: 31017468 Free PMC article.
-
Iron-Quercetin Complex Preconditioning of Human Peripheral Blood Mononuclear Cells Accelerates Angiogenic and Fibroblast Migration: Implications for Wound Healing.Int J Mol Sci. 2021 Aug 17;22(16):8851. doi: 10.3390/ijms22168851. Int J Mol Sci. 2021. PMID: 34445558 Free PMC article.
-
Effectiveness of quality and quantity mononuclear cells for enhancing wound healing in diabetic ischemic limb animal model.Int Wound J. 2025 Apr;22(4):e70106. doi: 10.1111/iwj.70106. Int Wound J. 2025. PMID: 40192089 Free PMC article.
-
Effect of Bacterial Nanocellulose with Chemisorbed Antiseptics on Alveolar Bone Repair in Rats Undergoing Bisphosphonate Therapy.Pharmaceutics. 2024 Dec 26;17(1):24. doi: 10.3390/pharmaceutics17010024. Pharmaceutics. 2024. PMID: 39861673 Free PMC article.
-
Reply to: Observation on the article "Long-term follow-up comparison of two different bilayer dermal substitutes in tissue regeneration: Clinical outcomes and histological findings".Int Wound J. 2020 Dec;17(6):1738-1739. doi: 10.1111/iwj.13383. Epub 2020 Jun 27. Int Wound J. 2020. PMID: 32592223 Free PMC article. No abstract available.
References
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–7. - PubMed
-
- Asahara T, Kawamoto A, Masuda H. Concise review: circulating endothelial progenitor cells for vascular medicine. Stem Cells. 2011;29(11):1650–5. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

