Aldose reductase inhibitor ranirestat significantly improves nerve conduction velocity in diabetic polyneuropathy: A randomized double-blind placebo-controlled study in Japan
- PMID: 29975462
- PMCID: PMC6400176
- DOI: 10.1111/jdi.12890
Aldose reductase inhibitor ranirestat significantly improves nerve conduction velocity in diabetic polyneuropathy: A randomized double-blind placebo-controlled study in Japan
Abstract
Aims/introduction: Diabetic polyneuropathy is one of the most frequent diabetic complications, and impairs patients' quality of life. We evaluated the efficacy and safety of ranirestat (40 mg/day) in patients with diabetic polyneuropathy.
Materials and methods: This was a multicenter, placebo-controlled, randomized double-blind, parallel-group, phase III study in which 557 patients were randomly assigned to either the ranirestat or placebo group and assessed for 52 weeks. The co-primary end-points were the changes in tibial motor nerve conduction velocity and total modified Toronto Clinical Neuropathy Score as a measure of clinical symptoms.
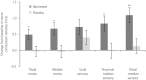
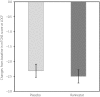
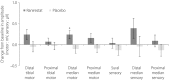
Results: There was a significant increase in tibial motor nerve conduction velocity in the ranirestat group compared with the placebo group. The difference between groups in the change at last observation was 0.52 m/s (P = 0.021). Increases in nerve conduction velocity in the ranirestat group were found not only in the tibial motor nerves, but also in the median motor nerves, proximal median sensory nerves and distal median sensory nerves. No significant differences in modified Toronto Clinical Neuropathy Score or safety parameters were found between the two groups.
Conclusions: Ranirestat (40 mg/day) was well tolerated and improved nerve conduction velocity. Regarding symptoms and signs, no detectable benefits over the placebo were observed in the ranirestat group during the 52 weeks of treatment.
Keywords: Diabetic polyneuropathy; Nerve conduction velocity; Ranirestat.
© 2018 The Authors Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Figures
Similar articles
-
Ranirestat for the management of diabetic sensorimotor polyneuropathy.Diabetes Care. 2009 Jul;32(7):1256-60. doi: 10.2337/dc08-2110. Epub 2009 Apr 14. Diabetes Care. 2009. PMID: 19366965 Free PMC article. Clinical Trial.
-
Long-term effects of ranirestat (AS-3201) on peripheral nerve function in patients with diabetic sensorimotor polyneuropathy.Diabetes Care. 2006 Jan;29(1):68-72. doi: 10.2337/diacare.29.01.06.dc05-1447. Diabetes Care. 2006. PMID: 16373898 Clinical Trial.
-
Effect of Ranirestat on Sensory and Motor Nerve Function in Japanese Patients with Diabetic Polyneuropathy: A Randomized Double-Blind Placebo-Controlled Study.J Diabetes Res. 2016;2016:5383797. doi: 10.1155/2016/5383797. Epub 2016 Jan 10. J Diabetes Res. 2016. PMID: 26881251 Free PMC article. Clinical Trial.
-
Evaluation of ranirestat for the treatment of diabetic neuropathy.Expert Opin Drug Metab Toxicol. 2014 Jul;10(7):1051-9. doi: 10.1517/17425255.2014.916277. Epub 2014 Apr 30. Expert Opin Drug Metab Toxicol. 2014. PMID: 24785785 Review.
-
Ranirestat as a therapeutic aldose reductase inhibitor for diabetic complications.Expert Opin Investig Drugs. 2008 Apr;17(4):575-81. doi: 10.1517/13543784.17.4.575. Expert Opin Investig Drugs. 2008. PMID: 18363521 Review.
Cited by
-
Lumos for the long trail: Strategies for clinical diagnosis and severity staging for diabetic polyneuropathy and future directions.J Diabetes Investig. 2020 Jan;11(1):5-16. doi: 10.1111/jdi.13173. Epub 2019 Dec 1. J Diabetes Investig. 2020. PMID: 31677343 Free PMC article. Review.
-
Aldose Reductase: a cause and a potential target for the treatment of diabetic complications.Arch Pharm Res. 2021 Jul;44(7):655-667. doi: 10.1007/s12272-021-01343-5. Epub 2021 Jul 19. Arch Pharm Res. 2021. PMID: 34279787 Review.
-
Ranirestat Improves Electrophysiologic but not Clinical Measures of Diabetic Polyneuropathy: A Meta-Analysis.Indian J Endocrinol Metab. 2022 Sep-Oct;26(5):399-406. doi: 10.4103/ijem.ijem_242_22. Epub 2022 Nov 22. Indian J Endocrinol Metab. 2022. PMID: 36618527 Free PMC article. Review.
-
Ranirestat Improved Nerve Conduction Velocities, Sensory Perception, and Intraepidermal Nerve Fiber Density in Rats with Overt Diabetic Polyneuropathy.J Diabetes Res. 2019 Nov 18;2019:2756020. doi: 10.1155/2019/2756020. eCollection 2019. J Diabetes Res. 2019. PMID: 31828158 Free PMC article.
-
Alpha-Tocopherol-Loaded Liposomes Reduce High Glucose Induced Oxidative Stress in Schwann Cells: A Proof of Concept Study.Diabetes Metab J. 2025 May;49(3):507-512. doi: 10.4093/dmj.2024.0489. Epub 2025 Feb 5. Diabetes Metab J. 2025. PMID: 39908988 Free PMC article.
References
-
- Vileikyte L, Rubin RR, Leventhal H. Psychological aspects of diabetic neuropathic foot complications: an overview. Diabetes Metab Res Rev 2004; 20: S13–S18. - PubMed
-
- Vincent AM, Callaghan BC, Smith AL, et al Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol 2011; 7: 573–583. - PubMed
-
- Dyck PJ, Zimmerman BR, Vilen TH, et al Nerve glucose, fructose, sorbitol, myo‐inositol, and fiber degeneration and regeneration in diabetic neuropathy. N Engl J Med 1988; 319: 542–548. - PubMed
-
- Greene DA, Arezzo JC, Brown MB, et al Effect of aldose reductase inhibition on nerve conduction and morphometry in diabetic neuropathy. Neurology 1999; 53: 580–591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

