Aldose reductase inhibitor ranirestat significantly improves nerve conduction velocity in diabetic polyneuropathy: A randomized double-blind placebo-controlled study in Japan
- PMID: 29975462
- PMCID: PMC6400176
- DOI: 10.1111/jdi.12890
Aldose reductase inhibitor ranirestat significantly improves nerve conduction velocity in diabetic polyneuropathy: A randomized double-blind placebo-controlled study in Japan
Abstract
Aims/introduction: Diabetic polyneuropathy is one of the most frequent diabetic complications, and impairs patients' quality of life. We evaluated the efficacy and safety of ranirestat (40 mg/day) in patients with diabetic polyneuropathy.
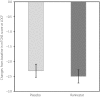
Materials and methods: This was a multicenter, placebo-controlled, randomized double-blind, parallel-group, phase III study in which 557 patients were randomly assigned to either the ranirestat or placebo group and assessed for 52 weeks. The co-primary end-points were the changes in tibial motor nerve conduction velocity and total modified Toronto Clinical Neuropathy Score as a measure of clinical symptoms.
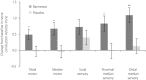
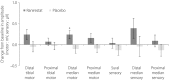
Results: There was a significant increase in tibial motor nerve conduction velocity in the ranirestat group compared with the placebo group. The difference between groups in the change at last observation was 0.52 m/s (P = 0.021). Increases in nerve conduction velocity in the ranirestat group were found not only in the tibial motor nerves, but also in the median motor nerves, proximal median sensory nerves and distal median sensory nerves. No significant differences in modified Toronto Clinical Neuropathy Score or safety parameters were found between the two groups.
Conclusions: Ranirestat (40 mg/day) was well tolerated and improved nerve conduction velocity. Regarding symptoms and signs, no detectable benefits over the placebo were observed in the ranirestat group during the 52 weeks of treatment.
Keywords: Diabetic polyneuropathy; Nerve conduction velocity; Ranirestat.
© 2018 The Authors Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Figures
References
-
- Vileikyte L, Rubin RR, Leventhal H. Psychological aspects of diabetic neuropathic foot complications: an overview. Diabetes Metab Res Rev 2004; 20: S13–S18. - PubMed
-
- Vincent AM, Callaghan BC, Smith AL, et al Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol 2011; 7: 573–583. - PubMed
-
- Dyck PJ, Zimmerman BR, Vilen TH, et al Nerve glucose, fructose, sorbitol, myo‐inositol, and fiber degeneration and regeneration in diabetic neuropathy. N Engl J Med 1988; 319: 542–548. - PubMed
-
- Greene DA, Arezzo JC, Brown MB, et al Effect of aldose reductase inhibition on nerve conduction and morphometry in diabetic neuropathy. Neurology 1999; 53: 580–591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

