A suboptimal maternal diet combined with accelerated postnatal growth results in an altered aging profile in the thymus of male rats
- PMID: 29975569
- PMCID: PMC6314471
- DOI: 10.1096/fj.201701350RR
A suboptimal maternal diet combined with accelerated postnatal growth results in an altered aging profile in the thymus of male rats
Abstract
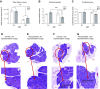
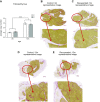
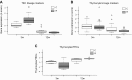
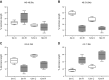
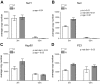
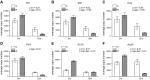
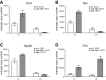
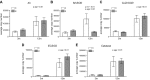
Reduced fetal nutrition and rapid postnatal growth accelerates the aging phenotype in many organ systems; however, effects on the immune system are unclear. We addressed this by studying the thymus from a rat model of developmental programming. The recuperated group was generated by in utero protein restriction, followed by cross-fostering to control-fed mothers, and were then compared with controls. Fat infiltration and adipocyte size increased with age ( P < 0.001) and in recuperated thymi ( P < 0.05). Cortex/medulla ratio decreased with age ( P < 0.001) and decreased ( P < 0.05) in 12-mo recuperated thymi. Age-associated decreases in thymic-epithelial cell ( P < 0.01) and thymocyte markers ( P < 0.01) were observed in both groups and was decreased ( P < 0.05) in recuperated thymi. These data demonstrate effects of developmental programming upon thymic involution. The recuperated group had longer thymic telomeres than controls ( P < 0.001) at 22 d and at 3 mo, which was associated with increased expression of telomere-length maintenance molecules [telomerase RNA component ( Terc; P < 0.01), P23 ( P = 0.02), and Ku70 and Ku80 ( P < 0.01)]. By 12 mo, recuperated offspring had shorter thymic telomeres than controls had ( P < 0.001) and reduced DNA damage-response markers [( DNA-PKcs, Mre11 ( P < 0.01), Xrcc4 ( P = 0.02), and γ-H2ax ( P < 0.001], suggesting failure of earlier compensatory responses. Our results suggest that low birth weight with rapid postnatal growth results in premature thymic maturation, resulting in accelerated thymic aging. This could lead to increased age-associated vulnerability to infection.-Tarry-Adkins, J. L., Aiken, C. E., Ashmore, T. J., Fernandez-Twinn, D. S., Chen, J.-H., Ozanne, S. E. A suboptimal maternal diet combined with accelerated postnatal growth results in an altered aging profile in the thymus of male rats.
Keywords: developmental programming; immunosenescence; involution.
Conflict of interest statement
The authors thank James Warner, Gregory Strachan, and M. S. Martin-Gronert (University of Cambridge Metaboloc Research Laboratories and MRC Metabolic Diseases Unit, Wellcome Trust-MRC Institute of Metabolic Science) for technical assistance. This work was supported by The British Heart Foundation (Grants PG/09/037/27387 and FS/09/029/27902), the Medical Research Council (MRC; Grant MC_UU_12012/4), and by an Isaac Newton Trust/Wellcome Institutional Strategic Support Fund (ISSF)/University of Cambridge Joint Research Grant. S.E.O. is a member of the MRC Metabolic Diseases Unit. The authors declare no conflicts of interest.
Figures


References
-
- Tarry-Adkins J. L., Ozanne S. E. (2017) Nutrition in early life and age-associated diseases. Ageing Res. Rev. 39, 96–105 - PubMed
-
- Aspinall R., Andrew D. (2000) Thymic involution in aging. J. Clin. Immunol. 20, 250–256 - PubMed
-
- Hales C. N., Barker D. J. (2001) The thrifty phenotype hypothesis. Br. Med. Bull. 60, 5–20 - PubMed
-
- Miller J. F. (1961) Immunological function of the thymus. Lancet 2, 748–749 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- PG/09/037/27387 /BHF_/British Heart Foundation/United Kingdom
- FS/09/029/27902/BHF_/British Heart Foundation/United Kingdom
- MC_UU_12012/4/MRC_/Medical Research Council/United Kingdom
- PG/09/037/27387/BHF_/British Heart Foundation/United Kingdom
- RG/17/12/33167/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

