Drosophila melanogaster establishes a species-specific mutualistic interaction with stable gut-colonizing bacteria
- PMID: 29975680
- PMCID: PMC6049943
- DOI: 10.1371/journal.pbio.2005710
Drosophila melanogaster establishes a species-specific mutualistic interaction with stable gut-colonizing bacteria
Abstract
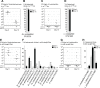
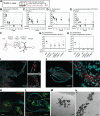
Animals live together with diverse bacteria that can impact their biology. In Drosophila melanogaster, gut-associated bacterial communities are relatively simple in composition but also have a strong impact on host development and physiology. It is generally assumed that gut bacteria in D. melanogaster are transient and their constant ingestion with food is required to maintain their presence in the gut. Here, we identify bacterial species from wild-caught D. melanogaster that stably associate with the host independently of continuous inoculation. Moreover, we show that specific Acetobacter wild isolates can proliferate in the gut. We further demonstrate that the interaction between D. melanogaster and the wild isolated Acetobacter thailandicus is mutually beneficial and that the stability of the gut association is key to this mutualism. The stable population in the gut of D. melanogaster allows continuous bacterial spreading into the environment, which is advantageous to the bacterium itself. The bacterial dissemination is in turn advantageous to the host because the next generation of flies develops in the presence of this particularly beneficial bacterium. A. thailandicus leads to a faster host development and higher fertility of emerging adults when compared to other bacteria isolated from wild-caught flies. Furthermore, A. thailandicus is sufficient and advantageous when D. melanogaster develops in axenic or freshly collected figs, respectively. This isolate of A. thailandicus colonizes several genotypes of D. melanogaster but not the closely related D. simulans, indicating that the stable association is host specific. This work establishes a new conceptual model to understand D. melanogaster-gut microbiota interactions in an ecological context; stable interactions can be mutualistic through microbial farming, a common strategy in insects. Moreover, these results develop the use of D. melanogaster as a model to study gut microbiota proliferation and colonization.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Metabolic Basis for Mutualism between Gut Bacteria and Its Impact on the Drosophila melanogaster Host.Appl Environ Microbiol. 2019 Jan 9;85(2):e01882-18. doi: 10.1128/AEM.01882-18. Print 2019 Jan 15. Appl Environ Microbiol. 2019. PMID: 30389767 Free PMC article.
-
The importance of being persistent: The first true resident gut symbiont in Drosophila.PLoS Biol. 2018 Aug 2;16(8):e2006945. doi: 10.1371/journal.pbio.2006945. eCollection 2018 Aug. PLoS Biol. 2018. PMID: 30071013 Free PMC article.
-
Spatiotemporally Heterogeneous Population Dynamics of Gut Bacteria Inferred from Fecal Time Series Data.mBio. 2018 Jan 9;9(1):e01453-17. doi: 10.1128/mBio.01453-17. mBio. 2018. PMID: 29317508 Free PMC article.
-
Links between the gut microbiota, metabolism, and host behavior.Gut Microbes. 2020;11(2):245-248. doi: 10.1080/19490976.2019.1643674. Epub 2019 Jul 25. Gut Microbes. 2020. PMID: 31345081 Free PMC article. Review.
-
Studying host-microbiota mutualism in Drosophila: Harnessing the power of gnotobiotic flies.Biomed J. 2015 Jul-Aug;38(4):285-93. doi: 10.4103/2319-4170.158620. Biomed J. 2015. PMID: 26068125 Review.
Cited by
-
Genome-Inferred Correspondence between Phylogeny and Metabolic Traits in the Wild Drosophila Gut Microbiome.Genome Biol Evol. 2021 Aug 3;13(8):evab127. doi: 10.1093/gbe/evab127. Genome Biol Evol. 2021. PMID: 34081101 Free PMC article.
-
Identification of key yeast species and microbe-microbe interactions impacting larval growth of Drosophila in the wild.Elife. 2023 Dec 27;12:RP90148. doi: 10.7554/eLife.90148. Elife. 2023. PMID: 38150375 Free PMC article.
-
Ribose-induced advanced glycation end products reduce the lifespan in Drosophila melanogaster by changing the redox state and down-regulating the Sirtuin genes.Biogerontology. 2024 Dec 19;26(1):28. doi: 10.1007/s10522-024-10172-0. Biogerontology. 2024. PMID: 39702854
-
Effects of Feeding Sources and Different Temperature Changes on the Gut Microbiome Structure of Chrysomya megacephala (Diptera: Calliphoridae).Insects. 2025 Mar 8;16(3):283. doi: 10.3390/insects16030283. Insects. 2025. PMID: 40266727 Free PMC article.
-
Metabolic cross-feeding in imbalanced diets allows gut microbes to improve reproduction and alter host behaviour.Nat Commun. 2020 Aug 25;11(1):4236. doi: 10.1038/s41467-020-18049-9. Nat Commun. 2020. PMID: 32843654 Free PMC article.
References
-
- Sasaki T, Ishikawa H. Production of Essential Amino Acids from Glutamate by Mycetocyte Symbionts of the Pea Aphid, Acyrthosiphon pisum. J Insect Physiol. 1995;41: 41–46.
-
- Douglas AE. Reproductive failure and the free amino acid pools in pea aphids (Acyrthosiphon pisum) lacking symbiotic bacteria. J Insect Physiol. 1996;42: 247–255. 10.1016/0022-1910(95)00105-0 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

