Paxillin phosphorylation at serine 273 and its effects on Rac, Rho and adhesion dynamics
- PMID: 29975690
- PMCID: PMC6053249
- DOI: 10.1371/journal.pcbi.1006303
Paxillin phosphorylation at serine 273 and its effects on Rac, Rho and adhesion dynamics
Abstract
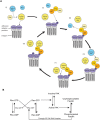
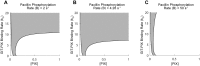
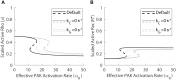
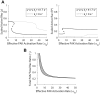
Focal adhesions are protein complexes that anchor cells to the extracellular matrix. During migration, the growth and disassembly of these structures are spatiotemporally regulated, with new adhesions forming at the leading edge of the cell and mature adhesions disassembling at the rear. Signalling proteins and structural cytoskeletal components tightly regulate adhesion dynamics. Paxillin, an adaptor protein within adhesions, is one of these proteins. Its phosphorylation at serine 273 (S273) is crucial for maintaining fast adhesion assembly and disassembly. Paxillin is known to bind to a GIT1-βPIX-PAK1 complex, which increases the local activation of the small GTPase Rac. To understand quantitatively the behaviour of this system and how it relates to adhesion assembly/disassembly, we developed a mathematical model describing the dynamics of the small GTPases Rac and Rho as determined by paxillin S273 phosphorylation. Our model revealed that the system possesses bistability, where switching between uninduced (active Rho) and induced (active Rac) states can occur through a change in rate of paxillin phosphorylation or PAK1 activation. The bistable switch is characterized by the presence of memory, minimal change in the levels of active Rac and Rho within the induced and uninduced states, respectively, and the limited regime of monostability associated with the uninduced state. These results were validated experimentally by showing the presence of bimodality in adhesion assembly and disassembly rates, and demonstrating that Rac activity increases after treating Chinese Hamster Ovary cells with okadaic acid (a paxillin phosphatase inhibitor), followed by a modest recovery after 20 min washout. Spatial gradients of phosphorylated paxillin in a reaction-diffusion model gave rise to distinct regions of Rac and Rho activities, resembling polarization of a cell into front and rear. Perturbing several parameters of the model also revealed important insights into how signalling components upstream and downstream of paxillin phosphorylation affect dynamics.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Paxillin S273 Phosphorylation Regulates Adhesion Dynamics and Cell Migration through a Common Protein Complex with PAK1 and βPIX.Sci Rep. 2019 Aug 7;9(1):11430. doi: 10.1038/s41598-019-47722-3. Sci Rep. 2019. PMID: 31391572 Free PMC article.
-
Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics.J Cell Biol. 2006 May 22;173(4):587-9. doi: 10.1083/jcb.200509075. J Cell Biol. 2006. PMID: 16717130 Free PMC article.
-
Polarity and mixed-mode oscillations may underlie different patterns of cellular migration.Sci Rep. 2023 Mar 14;13(1):4223. doi: 10.1038/s41598-023-31042-8. Sci Rep. 2023. PMID: 36918704 Free PMC article.
-
Paxillin comes of age.J Cell Sci. 2008 Aug 1;121(Pt 15):2435-44. doi: 10.1242/jcs.018044. J Cell Sci. 2008. PMID: 18650496 Free PMC article. Review.
-
Paxillin: a crossroad in pathological cell migration.J Hematol Oncol. 2017 Feb 18;10(1):50. doi: 10.1186/s13045-017-0418-y. J Hematol Oncol. 2017. PMID: 28214467 Free PMC article. Review.
Cited by
-
LncRNA CDKN2B-AS1/miR-141/cyclin D network regulates tumor progression and metastasis of renal cell carcinoma.Cell Death Dis. 2020 Aug 19;11(8):660. doi: 10.1038/s41419-020-02877-0. Cell Death Dis. 2020. PMID: 32814766 Free PMC article.
-
Targeting integrin pathways: mechanisms and advances in therapy.Signal Transduct Target Ther. 2023 Jan 2;8(1):1. doi: 10.1038/s41392-022-01259-6. Signal Transduct Target Ther. 2023. PMID: 36588107 Free PMC article. Review.
-
LncRNA MALAT1 facilitates BM-MSCs differentiation into endothelial cells and ameliorates erectile dysfunction via the miR-206/CDC42/PAK1/paxillin signalling axis.Reprod Biol Endocrinol. 2024 Jun 25;22(1):74. doi: 10.1186/s12958-024-01240-8. Reprod Biol Endocrinol. 2024. PMID: 38918809 Free PMC article.
-
Progesterone Receptor B signaling Reduces Breast Cancer Cell Aggressiveness: Role of Cyclin-D1/Cdk4 Mediating Paxillin Phosphorylation.Cancers (Basel). 2019 Aug 17;11(8):1201. doi: 10.3390/cancers11081201. Cancers (Basel). 2019. PMID: 31426542 Free PMC article.
-
Paxillin and Kindlin: Research Progress and Biological Functions.Biomolecules. 2025 Jan 24;15(2):173. doi: 10.3390/biom15020173. Biomolecules. 2025. PMID: 40001476 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

