Evolution of patients' socio-behavioral characteristics in the context of DAA: Results from the French ANRS CO13 HEPAVIH cohort of HIV-HCV co-infected patients
- PMID: 29975764
- PMCID: PMC6033422
- DOI: 10.1371/journal.pone.0199874
Evolution of patients' socio-behavioral characteristics in the context of DAA: Results from the French ANRS CO13 HEPAVIH cohort of HIV-HCV co-infected patients
Abstract
Background: Direct-acting antivirals (DAA) have dramatically increased HCV cure rates with minimal toxicity in HIV-HCV co-infected patients. This study aimed to compare the socio-behavioral characteristics of patients initiating pegylated-interferon (PEG-IFN)-based HCV treatment with those of patients initiating DAA-based treatment.
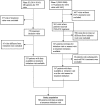
Methods: ANRS CO13 HEPAVIH is a national multicenter prospective cohort started in 2005, which enrolled 1,859 HIV-HCV co-infected patients followed up in French hospital outpatient units. Both clinical/biological and socio-behavioral data were collected during follow-up. We selected patients with socio-behavioral data available before HCV treatment initiation.
Results: A total of 580 patients were included in this analysis. Of these, 347 initiated PEG-IFN-based treatment, and 233 DAA-based treatment. There were significant differences regarding patient mean age (45 years±6 for the PEG-IFN group vs. 52 years±8 for the DAA group, p<0.001), unstable housing (21.4% vs. 11.2%, p = 0.0016), drug use (44.7% vs. 29.6%, p = 0.0003), regular or daily use of cannabis (24.3% vs. 15.6%, p = 0.0002), a history of drug injection (68.9% vs 39.0%, p<0.0001) and significant liver fibrosis (62.4% vs 72.3%, p = 0.0293). In multivariable analysis, patients initiating DAA-based treatment were older than their PEG-IFN-based treatment counterparts (aOR = 1.17; 95%CI [1.13; 1.22]). Patients receiving DAA treatment were less likely to report unstable housing (0.46 [0.24; 0.88]), cannabis use (regular or daily use:0.50 [0.28; 0.91]; non-regular use: 0.41 [0.22; 0.77]), and a history of drug injection (0.19 [0.12; 0.31]).
Conclusion: It is possible that a majority of patients who had socio-economic problems and/or a history of drug injection and/or a non-advanced disease stage were already treated for HCV in the PEG-IFN era. Today, patients with unstable housing conditions are prescribed DAA less frequently than other populations. As HCV treatment is prevention, improving access to DAA remains a major clinical and public health strategy, in particular for individuals with high-risk behaviors.
Conflict of interest statement
LW reports grants from ANRS, during the conduct of the study; grants from Inserm Aviesan, personal fees from BMS, Janssen, Gilead and MSD outside the submitted work; LP reports personal fees from Bristol Myers Squibb, Gilead, Viiv Healthcare, MSD, Pfizer, Gilead, Janssen Cilag, outside the submitted work. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
Similar articles
-
Efficacy and safety of direct-acting antiviral regimens in HIV/HCV-co-infected patients - French ANRS CO13 HEPAVIH cohort.J Hepatol. 2017 Jul;67(1):23-31. doi: 10.1016/j.jhep.2017.02.012. Epub 2017 Feb 22. J Hepatol. 2017. PMID: 28235612
-
All-oral Direct-acting Antiviral Regimens in HIV/Hepatitis C Virus-coinfected Patients With Cirrhosis Are Efficient and Safe: Real-life Results From the Prospective ANRS CO13-HEPAVIH Cohort.Clin Infect Dis. 2016 Sep 15;63(6):763-770. doi: 10.1093/cid/ciw379. Epub 2016 Jun 17. Clin Infect Dis. 2016. PMID: 27317796
-
Significant reductions in alcohol use after hepatitis C treatment: results from the ANRS CO13-HEPAVIH cohort.Addiction. 2017 Sep;112(9):1669-1679. doi: 10.1111/add.13851. Epub 2017 Jun 6. Addiction. 2017. PMID: 28430385
-
Direct-acting Antivirals for HIV/HCV Co-infected Individuals: As Good as it Gets?Curr HIV Res. 2017;15(6):422-433. doi: 10.2174/1570162X15666171108125255. Curr HIV Res. 2017. PMID: 29119934 Review.
-
Reactivation of occult HBV infection in an HIV/HCV Co-infected patient successfully treated with sofosbuvir/ledipasvir: a case report and review of the literature.BMC Infect Dis. 2017 Mar 1;17(1):182. doi: 10.1186/s12879-017-2287-y. BMC Infect Dis. 2017. PMID: 28249574 Free PMC article. Review.
Cited by
-
Alcohol Consumption and Risk of Liver Fibrosis in People Living With HIV: A Systematic Review and Meta-Analysis.Front Immunol. 2022 Mar 18;13:841314. doi: 10.3389/fimmu.2022.841314. eCollection 2022. Front Immunol. 2022. PMID: 35371091 Free PMC article.
References
-
- WHO | Hepatitis C [Internet]. WHO. [cited 2018 Jun 21]. Available from: http://www.who.int/news-room/fact-sheets/detail/hepatitis-c
-
- Zeuzem S, Berg T, Moeller B, Hinrichsen H, Mauss S, Wedemeyer H, et al. Expert opinion on the treatment of patients with chronic hepatitis C. J Viral Hepat. 2009;16:75–90. doi: 10.1111/j.1365-2893.2008.01012.x - DOI - PMC - PubMed
-
- Ward RP, Kugelmas M. Using pegylated interferon and ribavirin to treat patients with chronic hepatitis C. Am Fam Physician. 2005;72:655–62. - PubMed
-
- Lee SS, Bain VG, Peltekian K, Krajden M, Yoshida EM, Deschenes M, et al. Treating chronic hepatitis C with pegylated interferon alfa-2a (40 KD) and ribavirin in clinical practice. Aliment Pharmacol Ther. 2006;23:397–408. doi: 10.1111/j.1365-2036.2006.02748.x - DOI - PubMed
-
- Pawlotsky J-M. Hepatitis C treatment: the data flood goes on-an update from the liver meeting 2014. Gastroenterology. 2015;148:468–79. doi: 10.1053/j.gastro.2015.01.002 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials