Concise Synthesis of (-)-Cycloclavine and (-)-5- epi-Cycloclavine via Asymmetric C-C Activation
- PMID: 29976068
- PMCID: PMC6677407
- DOI: 10.1021/jacs.8b05549
Concise Synthesis of (-)-Cycloclavine and (-)-5- epi-Cycloclavine via Asymmetric C-C Activation
Abstract
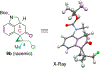
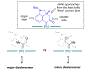
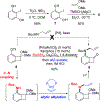
To illustrate the synthetic significance of C-C activation methods, here we describe an efficient strategy for the enantioselective total syntheses of (-)-cycloclavine and (-)-5- epi-cycloclavine, which is enabled by an asymmetric Rh-catalyzed "cut-and-sew" transformation between benzocyclobutenones and olefins. Despite the compact structure of cycloclavine with five-fused rings, the total synthesis was accomplished in 10 steps with a 30% overall yield. Key features of the synthesis include (1) a Pd-catalyzed tandem C-N bond coupling/allylic alkylation sequence to construct the nitrogen-tethered benzocyclobutenone, (2) a highly enantioselective Rh-catalyzed carboacylation of alkenes to forge the indoline-fused tricyclic structure, and (3) a diastereoselective cyclopropanation for preparing the tetrasubstituted cyclopropane ring. Notably, an improved catalytic condition has been developed for the nitrogen-tethered cut-and-sew transformation, which uses a low catalyst loading and allows for a broad substrate scope with high enantioselectivity (94-99% e.e.). The C-C activation-based strategy employed here is anticipated to have further implications for syntheses of other natural products that contain complex fused or bridged rings.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
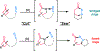
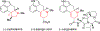



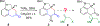
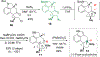
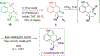

References
-
-
For selected reviews, see:
- Crabtree RH Chem. Rev 1985, 85, 245.
- Jones WD Nature 1993, 364, 676.
- Murakami M; Ito Y Top. Organomet. Chem 1999, 3, 97.
- Rybtchinski B; Milstein D Angew. Chem., Int. Ed 1999, 38, 870. - PubMed
- Jun C-H Chem. Soc. Rev 2004, 33, 610. - PubMed
- Necas D; Kotora M Curr. Org. Chem 2007, 11, 1566.
- Park YJ; Park J-W; Jun C-H Acc. Chem. Res 2008, 41, 222. - PubMed
- Murakami M; Matsuda T Chem. Commun 2011, 47, 1100. - PubMed
- Seiser T; Saget T; Tran DN; Cramer N Angew. Chem., Int. Ed 2011, 50, 7740. - PubMed
- Korotvicka A; Necas D; Kotora M Curr. Org. Chem 2012, 16, 1170.
- Chen F; Wang T; Jiao N Chem. Rev 2014, 114, 8613. - PubMed
- Dermenci A; Coe JW; Dong G Org. Chem. Front 2014, 1, 567. - PMC - PubMed
- Dong G C–C bond activation; Springer-Verlag: Berlin, 2014; Vol. 346.
- Murakami M; Ishida N Fundamental Reactions to Cleave Carbon–Carbon σ-Bonds with Transition Metal Complexes. In Cleavage of Carbon–Carbon Single Bonds by Transition Metals; Wiley-VCH Verlag GmbH & Co. KGaA: 2015; pp 1.
- Souillart L; Cramer N Chem. Rev 2015, 115, 9410. - PubMed
- Chen P.-h.; Dong G Chem. - Eur. J 2016, 22, 18290. - PMC - PubMed
- Chen P.-h.; Billett BA; Tsukamoto T; Dong G ACS Catal. 2017, 7, 1340. - PMC - PubMed
- Fumagalli G; Stanton S; Bower JF Chem. Rev 2017, 117, 9404. - PubMed
- Kim D-S; Park W-J; Jun C-H Chem. Rev 2017, 117, 8977. - PubMed
-
-
-
For selected reviews, see:
- Murakami M; Ishida N Total Syntheses of Natural Products and Biologically Active Compounds by Transition-Metal-Catalyzed C–C Cleavage. In Cleavage of Carbon-Carbon Single Bonds by Transition Metals; Wiley-VCH Verlag GmbH & Co. KGaA: 2015; pp 253. For selected examples, see:
- South MS; Liebeskind LS J. Am. Chem. Soc 1984, 106, 4181.
- Wender PA; Fuji M; Husfeld CO; Love JA Org. Lett 1999, 1, 137.
- Trost BM; Waser J; Meyer AJ Am. Chem. Soc 2007, 129, 14556. - PMC - PubMed
- Jiao L; Yuan C; Yu Z-XJ Am. Chem. Soc 2008, 130, 4421. - PubMed
- Nakao Y; Ebata S; Yada A; Hiyama T; Ikawa M; Ogoshi SJ Am. Chem. Soc 2008, 130, 12874. - PubMed
- Hirata Y; Yukawa T; Kashihara N; Nakao Y; Hiyama TJ Am. Chem. Soc 2009, 131, 10964. - PubMed
- Liang Y; Jiang X; Yu Z-X Chem. Commun 2011, 47, 6659. - PubMed
- Evans PA; Inglesby PA; Kilbride K Org. Lett 2013, 15, 1798. - PubMed
- Xu T; Dong G Angew. Chem., Int. Ed 2014, 53, 10733. - PMC - PubMed
- Sun T; Zhang Y; Qiu B; Wang Y; Qin Y; Dong G; Xu T Angew. Chem., Int. Ed 2018, 57, 2859. - PubMed
-
-
- Xu T; Dong G Angew. Chem., Int. Ed 2012, 51, 7567. - PubMed
-
- Souillart L; Parker E; Cramer N Asymmetric Transformations via C–C Bond Cleavage In C–C Bond Activation; Dong G, Ed.; Springer: Berlin Heidelberg, 2014; pp 163. - PubMed
-
- For asymmetric C–C activation reactions via oxidative addition, see:
- Xu T; Ko HM; Savage NA; Dong GJ Am. Chem. Soc 2012, 134, 20005. - PubMed
- Parker E; Cramer N Organometallics 2014, 33, 780.
- Souillart L; Cramer N Angew. Chem., Int. Ed 2014, 53, 9640. - PubMed
- Souillart L; Parker E; Cramer N Angew. Chem., Int. Ed 2014, 53, 3001. - PubMed
- Zhou X; Dong GJ Am. Chem. Soc 2015, 137, 13715. - PMC - PubMed
- Deng L; Xu T; Li H; Dong GJ Am. Chem. Soc 2016, 138, 369. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

