Diversity, evolution, and function of myriapod hemocyanins
- PMID: 29976142
- PMCID: PMC6034248
- DOI: 10.1186/s12862-018-1221-2
Diversity, evolution, and function of myriapod hemocyanins
Abstract
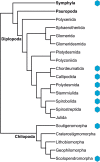
Background: Hemocyanin transports O2 in the hemolymph of many arthropod species. Such respiratory proteins have long been considered unnecessary in Myriapoda. As a result, the presence of hemocyanin in Myriapoda has long been overlooked. We analyzed transcriptome and genome sequences from all major myriapod taxa - Chilopoda, Diplopoda, Symphyla, and Pauropoda - with the aim of identifying hemocyanin-like proteins.
Results: We investigated the genomes and transcriptomes of 56 myriapod species and identified 46 novel full-length hemocyanin subunit sequences in 20 species of Chilopoda, Diplopoda, and Symphyla, but not Pauropoda. We found in Cleidogona sp. (Diplopoda, Chordeumatida) a hemocyanin-like sequence with mutated copper-binding centers, which cannot bind O2. An RNA-seq approach showed markedly different hemocyanin mRNA levels from ~ 6 to 25,000 reads per kilobase per million reads. To evaluate the contribution of hemocyanin to O2 transport, we specifically studied the hemocyanin of the centipede Scolopendra dehaani. This species harbors two distinct hemocyanin subunits with low expression levels. We showed cooperative O2 binding in the S. dehaani hemolymph, indicating that hemocyanin supports O2 transport even at low concentration. Further, we demonstrated that hemocyanin is > 1500-fold more highly expressed in the fertilized egg than in the adult.
Conclusion: Hemocyanin was most likely the respiratory protein in the myriapod stem-lineage, but multiple taxa may have independently lost hemocyanin and thus the ability of efficient O2 transport. In myriapods, hemocyanin is much more widespread than initially appreciated. Some myriapods express hemocyanin only at low levels, which are, nevertheless, sufficient for O2 supply. Notably, also in myriapods, a non-respiratory protein similar to insect storage hexamerins evolved from the hemocyanin.
Keywords: Arthropoda; Evolution; Hemocyanin; Myriapoda; Phenoloxidase; Subunit diversity.
Conflict of interest statement
Ethics approval and consent to participate
We followed the regulations of the German Animal Welfare Act (TierSchG).
Consent for publication
Does not apply.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Brusca RC, Brusca GJ. Invertebrates. Sinauer: Sunderland; 2003.
-
- Mangum CP. Oxygen transport in invertebrates. Am J Phys. 1985;248(5 Pt 2):R505–R514. - PubMed
-
- Markl J. Evolution and function of structurally diverse subunits in the respiratory protein hemocyanin from arthropods. Biol Bull. 1986;171:90–115. doi: 10.2307/1541909. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

