3-O-Acetyloleanolic acid inhibits VEGF-A-induced lymphangiogenesis and lymph node metastasis in an oral cancer sentinel lymph node animal model
- PMID: 29976150
- PMCID: PMC6034267
- DOI: 10.1186/s12885-018-4630-0
3-O-Acetyloleanolic acid inhibits VEGF-A-induced lymphangiogenesis and lymph node metastasis in an oral cancer sentinel lymph node animal model
Abstract
Background: Sentinel lymph node metastasis is a common and early event in the metastatic process of head and neck squamous cell carcinoma (HNSCC) and is the most powerful prognostic factor for survival of HNSCC patients. 3-O-acetyloleanolic acid (3AOA), a pentacyclic triterpenoid compound isolated from seeds of Vigna sinensis K., has been reported to have potent anti-angiogenesis and anti-tumor activities. However, its effects on tumor-related lymphangiogenesis and lymph node metastasis are not yet understood.
Methods: The in vitro inhibitory effects of 3AOA on VEGF-A-induced lymphangiogenesis were investigated via in vitro experiments using mouse oral squamous cell carcinoma (SCCVII) cells and human lymphatic microvascular endothelial cells (HLMECs). The in vivo inhibitory effects of 3AOA on VEGF-A-induced lymphangiogenesis and sentinel lymph node metastasis were investigated in an oral cancer sentinel lymph node (OCSLN) animal model.
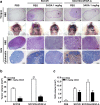
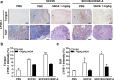
Results: 3AOA inhibited tumor-induced lymphangiogenesis and sentinel lymph node metastasis in an OCSLN animal model, and reduced expression of VEGF-A, a lymphangiogenic factor in hypoxia mimetic agent CoCl2-treated SCCVII cells. 3AOA inhibited proliferation, tube formation, and migration of VEGF-A-treated HLMECs. The lymphatic vessel formation that was stimulated in vivo in a by VEGF-A Matrigel plug was reduced by 3AOA. 3AOA suppressed phosphorylation of vascular endothelial growth factor (VEGFR) -1 and - 2 receptors that was stimulated by VEGF-A. In addition, 3AOA suppressed phosphorylation of the lymphangiogenesis-related downstream signaling factors PI3K, FAK, AKT, and ERK1/2. 3AOA inhibited tumor growth, tumor-induced lymphangiogenesis, and sentinel lymph node metastasis in a VEGF-A-induced OCSLN animal model that was established using VEGF-A overexpressing SCCVII cells.
Conclusion: 3AOA inhibits VEGF-A-induced lymphangiogenesis and sentinel lymph node metastasis both in vitro and in vivo. The anti-lymphangiogenic effects of 3AOA are probably mediated via suppression of VEGF-A/VEGFR-1 and VEGFR-2 signaling in HLMECs, and can be a useful anti-tumor agent to restrict the metastatic spread of oral cancer.
Keywords: 3-O-acetyloleanolic acid; Lymph node metastasis; Lymphangiogenesis; Oral cancer sentinel lymph node animal model; VEGF-A.
Conflict of interest statement
Ethics approval
This study (KHUASP-15-09) was reviewed and approved by the Institutional Animal Care and Use Committee of Kyung Hee University, and animal care and experimental procedures followed the University guidelines for the care and use of laboratory animals. The human cell line, HLMEC cells used in this study, were purchased from Lonza, and the use of widely available cell lines (Lonza, a publicly accessible repository) does not require ethical approval at our institution.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Baek CH. Sentinel lymph node biopsy in the oral cavity cancer. Hanyang Med Rev. 2009;29(3):255–264. doi: 10.7599/hmr.2009.29.3.255. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

