Docetaxel facilitates lymphatic-tumor crosstalk to promote lymphangiogenesis and cancer progression
- PMID: 29976154
- PMCID: PMC6034223
- DOI: 10.1186/s12885-018-4619-8
Docetaxel facilitates lymphatic-tumor crosstalk to promote lymphangiogenesis and cancer progression
Abstract
Background: Infiltration into lymphatic vessels is a critical step in breast cancer metastasis. Lymphatics undergo changes that facilitate metastasis as a result of activation of the cells lining lymphatic vessels, lymphatic endothelial cells (LECs). Inhibition of activation by targeting VEGFR3 can reduce invasion toward lymphatics. To best benefit patients, this approach should be coupled with standard of care that slows tumor growth, such as chemotherapy. Little is known about how chemotherapies, like docetaxel, may influence lymphatics and conversely, how lymphatics can alter responses to therapy.
Methods: A novel 3D in vitro co-culture model of the human breast tumor microenvironment was employed to examine the contribution of LECs to tumor invasion and viability with docetaxel and anti-VEGFR3, using three cell lines, MDA-MB-231, HCC38, and HCC1806. In vivo, the 4T1 mouse model of breast carcinoma was used to examine the efficacy of combinatorial therapy with docetaxel and anti-VEGFR3 on lymph node metastasis and tumor growth. Lymphangiogenesis in these mice was analyzed by immunohistochemistry and flow cytometry. Luminex analysis was used to measure expression of lymphangiogenic cytokines.
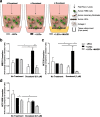
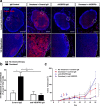
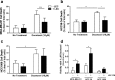
Results: In vitro, tumor cell invasion significantly increased with docetaxel when LECs were present; this effect was attenuated by inhibition of VEGFR3. LECs reduced docetaxel-induced cell death independent of VEGFR3. In vivo, docetaxel significantly increased breast cancer metastasis to the lymph node. Docetaxel and anti-VEGFR3 combination therapy reduced lymph node and lung metastasis in 4T1 and synergized to reduce tumor growth. Docetaxel induced VEGFR3-dependent vessel enlargement, lymphangiogenesis, and expansion of the LEC population in the peritumoral microenvironment, but not tumor-free stroma. Docetaxel caused an upregulation in pro-lymphangiogenic factors including VEGFC and TNF-α in the tumor microenvironment in vivo.
Conclusions: Here we present a counter-therapeutic effect of docetaxel chemotherapy that triggers cancer cells to elicit lymphangiogenesis. In turn, lymphatics reduce cancer response to docetaxel by altering the cytokine milieu in breast cancer. These changes lead to an increase in tumor cell invasion and survival under docetaxel treatment, ultimately reducing docetaxel efficacy. These docetaxel-induced effects can be mitigated by anti-VEGFR3 therapy, resulting in a synergism between these treatments that reduces tumor growth and metastasis.
Keywords: Cancer cell invasion; Docetaxel; Lymphangiogenesis; Taxane chemotherapy; Tissue engineered cell culture models; Triple-negative breast cancer; Tumor microenvironment; Tumor-associated lymphatics; VEGFC/VEGFR3.
Conflict of interest statement
Ethics approval
The animal care facilities and programs at the University of Virginia meet the requirements of the law and NIH regulations. The UVA vivariums are fully AAALAC accredited facilities. These are overseen by a full-time veterinarian. It is a barrier facility with HEPA-filtered air and autoclaved food and bedding is available. 24 h monitoring and care of the animals is provided by the staff. The Munson lab holds an ACUC approval for breast cancer related work, Protocol 0489 for a three-year period beginning August 2015. All of our cell lines were commercially purchased and therefore did not require ethics approval for our use.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Vascular endothelial growth factor C disrupts the endothelial lymphatic barrier to promote colorectal cancer invasion.Gastroenterology. 2015 Jun;148(7):1438-51.e8. doi: 10.1053/j.gastro.2015.03.005. Epub 2015 Mar 6. Gastroenterology. 2015. PMID: 25754161
-
Suppression of vascular endothelial growth factor receptor 3 (VEGFR3) and vascular endothelial growth factor C (VEGFC) inhibits hypoxia-induced lymph node metastases in cervix cancer.Gynecol Oncol. 2011 Nov;123(2):393-400. doi: 10.1016/j.ygyno.2011.07.006. Epub 2011 Aug 12. Gynecol Oncol. 2011. PMID: 21839498
-
Targeting VEGFR-3/-2 signaling pathways with AD0157: a potential strategy against tumor-associated lymphangiogenesis and lymphatic metastases.J Hematol Oncol. 2017 Jun 19;10(1):122. doi: 10.1186/s13045-017-0484-1. J Hematol Oncol. 2017. PMID: 28629427 Free PMC article.
-
Pathway-related molecules of VEGFC/D-VEGFR3/NRP2 axis in tumor lymphangiogenesis and lymphatic metastasis.Clin Chim Acta. 2016 Oct 1;461:165-71. doi: 10.1016/j.cca.2016.08.008. Epub 2016 Aug 12. Clin Chim Acta. 2016. PMID: 27527412 Review.
-
Two Birds, One Stone: Double Hits on Tumor Growth and Lymphangiogenesis by Targeting Vascular Endothelial Growth Factor Receptor 3.Cells. 2019 Mar 21;8(3):270. doi: 10.3390/cells8030270. Cells. 2019. PMID: 30901976 Free PMC article. Review.
Cited by
-
Effect of crosstalk among conspirators in tumor microenvironment on niche metastasis of gastric cancer.Am J Cancer Res. 2022 Dec 15;12(12):5375-5402. eCollection 2022. Am J Cancer Res. 2022. PMID: 36628284 Free PMC article. Review.
-
Unveiling the immunomodulatory dance: endothelial cells' function and their role in non-small cell lung cancer.Mol Cancer. 2025 Jan 16;24(1):21. doi: 10.1186/s12943-024-02221-6. Mol Cancer. 2025. PMID: 39819502 Free PMC article. Review.
-
VEGFC negatively regulates the growth and aggressiveness of medulloblastoma cells.Commun Biol. 2020 Oct 16;3(1):579. doi: 10.1038/s42003-020-01306-4. Commun Biol. 2020. PMID: 33067561 Free PMC article.
-
Modeling Immunity In Vitro: Slices, Chips, and Engineered Tissues.Annu Rev Biomed Eng. 2021 Jul 13;23:461-491. doi: 10.1146/annurev-bioeng-082420-124920. Epub 2021 Apr 19. Annu Rev Biomed Eng. 2021. PMID: 33872520 Free PMC article.
-
VEGFR3 tyrosine kinase inhibition aggravates cisplatin nephrotoxicity.Am J Physiol Renal Physiol. 2021 Dec 1;321(6):F675-F688. doi: 10.1152/ajprenal.00186.2021. Epub 2021 Oct 18. Am J Physiol Renal Physiol. 2021. PMID: 34658261 Free PMC article.
References
-
- Balko JM, et al. Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic targets. Cancer Discov. 2014;4:232–245. doi: 10.1158/2159-8290.CD-13-0286. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

