Promoting quality use of medicines in South-East Asia: reports from country situational analyses
- PMID: 29976180
- PMCID: PMC6034320
- DOI: 10.1186/s12913-018-3333-1
Promoting quality use of medicines in South-East Asia: reports from country situational analyses
Abstract
Background: Irrational use of medicines is widespread in the South-East Asia Region (SEAR), where policy implementation to encourage quality use of medicines (QUM) is often low. The aim was to determine whether public-sector QUM is better in SEAR countries implementing essential medicines (EM) policies than in those not implementing them.
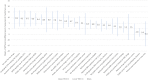
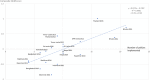
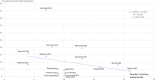
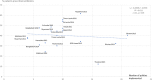
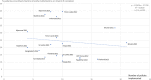
Methods: Data on six QUM indicators and 25 EM policies were extracted from situational analysis reports of 20 country (2-week) visits made during 2010-2015. The average difference (as percent) for the QUM indicators between countries implementing versus not implementing specific policies was calculated. Policies associated with better (> 1%) QUM were included in regression of a composite QUM score versus total number of policies implemented.
Results: Twenty-two policies were associated with better (> 1%) QUM. Twelve policies were associated with 3.6-9.5% significantly better use (p < 0.05), namely: standard treatment guidelines; formulary; a government unit to promote QUM; continuing health worker education on prescribing by government; limiting over-the-counter (OTC) availability of systemic antibiotics; disallowing public-sector prescriber revenue from medicines sales; not charging fees at the point of care; monitoring advertisements of OTC medicines; public education on QUM; and a good drug supply system. There was significant correlation between the number of policies implemented out of 22 and the composite QUM score (r = 0.71, r2 = 0.50, p < 0.05).
Conclusions: Country situational analyses allowed rapid data collection that showed EM policies are associated with better QUM. SEAR countries should implement all such policies.
Keywords: Essential medicines policy; Quality use of medicines; South-East Asia.
Conflict of interest statement
Ethics approval and consent to participate
All the data used in this study was obtained from published country reports of situational analyses of medicines management - all of which are publicly available online at:
All administrative permissions, including formal approval by the respective Ministry’s of Health were obtained, prior to publication of the country reports on the WHO/SEARO website, as mandated by the World Health Organisation’s Regional Committee Resolution SEA/RC66/R7, Effective Management of Medicine, World Health Organization Regional Office for South-East Asia, New Delhi, 2013 (see reference [38]). Furthermore, various data from these country reports have already been extracted, analysed, and published on the WHO/SEARO website and internationally (see reference [22]).
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Holloway KA, van Dijk L. The world medicines situation. Rational use of medicines. WHO/EMP/MIE/2011.2.2. 3. Geneva: World Health Organization; 2011.
-
- World Health Organisation . Medicines use in primary care in developing and transitional countries: fact book summarizing results from studies reported between 1990 and 2006. WHO/EMP/MAR/2009.3. Geneva: World Health Organisation; 2009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

