Assessing Plasmodium falciparum transmission in mosquito-feeding assays using quantitative PCR
- PMID: 29976199
- PMCID: PMC6034226
- DOI: 10.1186/s12936-018-2382-6
Assessing Plasmodium falciparum transmission in mosquito-feeding assays using quantitative PCR
Abstract
Background: Evaluating the efficacy of transmission-blocking interventions relies on mosquito-feeding assays, with transmission typically assessed by microscopic identification of oocysts in mosquito midguts; however, microscopy has limited throughput, sensitivity and specificity. Where low prevalence and intensity mosquito infections occur, as observed during controlled human malaria infection studies or natural transmission, a reliable method for detection and quantification of low-level midgut infection is required. Here, a semi-automated, Taqman quantitative PCR (qPCR) assay sufficiently sensitive to detect a single-oocyst midgut infection is described.
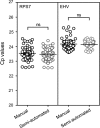
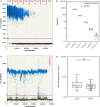
Results: Extraction of genomic DNA from Anopheles stephensi midguts using a semi-automated extraction process was shown to have equivalent extraction efficiency to manual DNA extraction. An 18S Plasmodium falciparum qPCR assay was adapted for quantitative detection of P. falciparum midgut oocyst infection using synthetic DNA standards. The assay was validated for sensitivity and specificity, and the limit of detection was 0.7 genomes/µL (95% CI 0.4-1.6 genomes/µL). All microscopy-confirmed oocyst infected midgut samples were detected by qPCR, including all single-oocyst positive midguts. The genome number per oocyst was assessed 8-9 days after feeding assay using both qPCR and droplet digital PCR and was 3722 (IQR: 2951-5453) and 3490 (IQR: 2720-4182), respectively.
Conclusions: This semi-automated qPCR method enables accurate detection of low-level P. falciparum oocyst infections in mosquito midguts, and may improve the sensitivity, specificity and throughput of assays used to evaluate candidate transmission-blocking interventions.
Keywords: Droplet digital PCR; Malaria; Oocyst; PCR; Plasmodium falciparum; Taqman; Transmission; Transmission-blocking; qPCR.
Figures
References
-
- WHO . World malaria report. Geneva: World Health Organization; 2016.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

