Patient-reported outcome measures used in patients with primary sclerosing cholangitis: a systematic review
- PMID: 29976215
- PMCID: PMC6034220
- DOI: 10.1186/s12955-018-0951-6
Patient-reported outcome measures used in patients with primary sclerosing cholangitis: a systematic review
Abstract
Background: Primary Sclerosing Cholangitis (PSC) is a rare chronic, cholestatic liver condition in which patients can experience a range of debilitating symptoms. Patient reported outcome measures (PROMs) could provide a valuable insight into the impact of PSC on patient quality of life and symptoms. A previous review has been conducted on the quality of life instruments used in liver transplant recipients. However, there has been no comprehensive review evaluating PROM use or measurement properties in PSC patients' to-date. The aim of the systematic review was to: (a) To identify and categorise which PROMs are currently being used in research involving the PSC population (b) To investigate the measurement properties of PROMs used in PSC.
Methods: A systematic review of Medline, EMBASE and CINAHL, from inception to February 2018, was undertaken. The methodological quality of included studies was assessed using the Consensus-based Standards for selection of health Measurement Instruments (COSMIN) checklist.
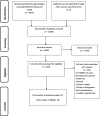
Results: Thirty-seven studies were identified, which included 36 different PROMs. Seven PROMs were generic, 10 disease-specific, 17 symptom-specific measures and 2 measures on dietary intake. The most common PROMs were the Short form-36 (SF-36) (n = 15) and Chronic liver disease questionnaire (CLDQ) (n = 6). Only three studies evaluated measurement properties, two studies evaluated the National Institute of Diabetes Digestive and Kidney Diseases Liver Transplant (NIDDK-QA) and one study evaluated the PSC PRO; however, according to the COSMIN guidelines, methodological quality was poor for the NIDDK-QA studies and fair for the PSC PRO study.
Conclusion: A wide variety of PROMs have been used to assess health-related quality of life and symptom burden in patients with PSC; however only two measures (NIDDK-QA and PSC PRO) have been formally validated in this population. The newly developed PSC PRO requires further validation in PSC patients with diverse demographics, comorbidities and at different stages of disease; however this is a promising new measure with which to assess the impact of PSC on patient quality of life and symptoms.
Keywords: Cholestasis; PROSPERO (Registration Number: CRD42016036544).; Patient reported outcome measures (PROMs); Primary sclerosing cholangitis.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Evaluating measures of quality of life in adult scoliosis: a systematic review and narrative synthesis.Spine Deform. 2022 Sep;10(5):991-1002. doi: 10.1007/s43390-022-00498-5. Epub 2022 Mar 29. Spine Deform. 2022. PMID: 35349122
-
Patient-reported outcome measures for assessing health-related quality of life in people with type 2 diabetes: A systematic review.Rev Endocr Metab Disord. 2022 Oct;23(5):931-977. doi: 10.1007/s11154-022-09734-9. Epub 2022 Jul 2. Rev Endocr Metab Disord. 2022. PMID: 35779199 Free PMC article.
-
HIV-Specific Reported Outcome Measures: Systematic Review of Psychometric Properties.JMIR Public Health Surveill. 2022 Dec 8;8(12):e39015. doi: 10.2196/39015. JMIR Public Health Surveill. 2022. PMID: 36222289 Free PMC article.
-
Comparison of cellulose, modified cellulose and synthetic membranes in the haemodialysis of patients with end-stage renal disease.Cochrane Database Syst Rev. 2001;(3):CD003234. doi: 10.1002/14651858.CD003234. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2005 Jul 20;(3):CD003234. doi: 10.1002/14651858.CD003234.pub2. PMID: 11687058 Updated.
-
Patient-Reported Outcome Measures in Carotid Artery Revascularization: Systematic Review and Psychometric Analysis.Ann Vasc Surg. 2018 Jul;50:275-283. doi: 10.1016/j.avsg.2017.12.008. Epub 2018 Mar 6. Ann Vasc Surg. 2018. PMID: 29501592
Cited by
-
European Society for Organ Transplantation (ESOT) Consensus Statement on Outcome Measures in Liver Transplantation According to Value-Based Health Care.Transpl Int. 2024 Jan 25;36:12190. doi: 10.3389/ti.2023.12190. eCollection 2023. Transpl Int. 2024. PMID: 38332850 Free PMC article.
-
A systematic review assessing the quality of patient reported outcomes measures in dry eye diseases.PLoS One. 2021 Aug 9;16(8):e0253857. doi: 10.1371/journal.pone.0253857. eCollection 2021. PLoS One. 2021. PMID: 34370748 Free PMC article.
-
Quality of life in primary sclerosing cholangitis: a systematic review.Health Qual Life Outcomes. 2021 Mar 20;19(1):100. doi: 10.1186/s12955-021-01739-3. Health Qual Life Outcomes. 2021. PMID: 33743710 Free PMC article.
-
Primary sclerosing cholangitis.Nat Rev Dis Primers. 2025 Mar 13;11(1):17. doi: 10.1038/s41572-025-00600-x. Nat Rev Dis Primers. 2025. PMID: 40082445 Review.
-
Patient and clinician opinions of patient reported outcome measures (PROMs) in the management of patients with rare diseases: a qualitative study.Health Qual Life Outcomes. 2020 Jun 10;18(1):177. doi: 10.1186/s12955-020-01438-5. Health Qual Life Outcomes. 2020. PMID: 32522194 Free PMC article.
References
-
- Primary Sclerosing Cholangitis [http://rarediseases.org/rare-diseases/primary-sclerosing-cholangitis/].
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources