Assessing patients' acceptance of their medication to reveal unmet needs: results from a large multi-diseases study using a patient online community
- PMID: 29976222
- PMCID: PMC6034222
- DOI: 10.1186/s12955-018-0962-3
Assessing patients' acceptance of their medication to reveal unmet needs: results from a large multi-diseases study using a patient online community
Abstract
Background: Patients with chronic conditions are required to take long-term treatments for their disease itself or to prevent any potential health risks. Measuring patient acceptance of their medication should help to better understand and predict patients' behavior toward treatment. This study aimed to describe the level of patient acceptance toward various long-term treatments in real life using an online patient community.
Methods: This was an observational, cross-sectional study conducted through the French Carenity platform. All Carenity patient members were invited to complete an online questionnaire including the 25-item ACCEptance by the Patients of their Treatment (ACCEPT©) questionnaire. ACCEPT© measures patient acceptance toward their medication and includes one general acceptance dimension (Acceptance/General) and six treatment-attribute specific dimensions (scores 0-100; lowest to highest acceptance): Acceptance/Medication Inconvenience, Acceptance/Long-term Treatment, Acceptance/Regimen Constraints, Acceptance/Side effects, Acceptance/Effectiveness, and Acceptance/Numerous Medications. Patients included in the analysis were treated adults experiencing any chronic diseases and who responded to at least one ACCEPT© item.
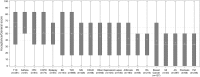
Results: Among the 4193 patients included in the analysis, more than 270 chronic diseases were represented, amidst which 19 included more than 30 patients. Mean ACCEPT© Acceptance/General score for those 19 diseases were 61.2 (SD = 31.9) for type 1 diabetes, 59.8 (SD = 32.3) for asthma, 56.3 (SD = 34.3) for hypertension, 52.0 (SD = 32.2) for chronic obstructive pulmonary disease, 51.7 (SD = 27.0) for epilepsy, 50.1 (SD = 33.1) for bipolar disorder, 49.9 (SD = 33.1) for type 2 diabetes, 48.6 (SD = 31.6) for multiple sclerosis, 46.1 (SD = 34.5) for Crohn's disease/ulcerative colitis, 44.3 (SD = 31.5) for depression, 42.8 (SD = 31.5) for lupus, 42.3 (SD = 33.0) for arthrosis, 41.8 (SD = 32.6) for Parkinson's disease, 40.5 (SD = 32.2) for rheumatoid arthritis, 38.6 (SD = 31.7) for breast cancer, 36.4 (SD = 36.4) for myocardial infarction, 35.8 (SD = 32.0) for ankylosing spondylitis, 34.1 (SD = 32.3) for psoriasis, and 33.7 (SD = 31.7) for fibromyalgia.
Conclusions: This first of its kind study enabled ACCEPT© data to be collected in real life for a variety of chronic diseases. These data may help in evaluating and interpreting levels of acceptance in future studies and provide valuable insights about patient priorities and current unmet needs.
Keywords: Acceptance; Chronic disease; Convenience; Observational study; Questionnaire; Risk/benefit assessment; Tolerability.
Conflict of interest statement
Ethics approval and consent to participate
As members of the Carenity platform, patients involved in this study were informed as they registered on the platform, and have consented expressly and specifically to the collection, handling and keeping of their personal and health data. They were also informed before starting the survey of the purpose of the study. The procedures followed for this study were in accordance with the French Law 78–17 of 6 January 1978 and its later amendments and with the 1964 Helsinki declaration and its later amendments.
Consent for publication
Not applicable.
Competing interests
BA, HG and JL were employees of Mapi at the time of the study. CA was a consultant, now employee of Mapi. MC is founder and CEO of Carenity.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Item Reduction, Scoring, and First Validation of the ACCEPTance by the Patients of their Treatment (ACCEPT©) Questionnaire.Patient. 2017 Feb;10(1):81-92. doi: 10.1007/s40271-016-0187-7. Patient. 2017. PMID: 27456210
-
Chronic patients' satisfaction and priorities regarding medical care, information and services and quality of life: a French online patient community survey.BMC Health Serv Res. 2020 Jun 5;20(1):511. doi: 10.1186/s12913-020-05373-5. BMC Health Serv Res. 2020. PMID: 32503523 Free PMC article.
-
Long-term treatment acceptance: what is it, and how can it be assessed?Patient. 2012;5(4):239-49. doi: 10.2165/11631340-000000000-00000. Patient. 2012. PMID: 23116323
-
Development and validation of the "Treatment Satisfaction with Medicines Questionnaire" (SATMED-Q).Value Health. 2008 Sep-Oct;11(5):913-26. doi: 10.1111/j.1524-4733.2008.00323.x. Epub 2008 May 20. Value Health. 2008. PMID: 18494753
-
The acceptability of the risk of death in the treatment of respiratory diseases in France.Health Econ Rev. 2024 Oct 1;14(1):78. doi: 10.1186/s13561-024-00541-3. Health Econ Rev. 2024. PMID: 39352608 Free PMC article. Review.
Cited by
-
Intramuscular versus enteral penicillin prophylaxis to prevent progression of rheumatic heart disease: Study protocol for a noninferiority randomized trial (the GOALIE trial).Am Heart J. 2024 Sep;275:74-85. doi: 10.1016/j.ahj.2024.05.012. Epub 2024 May 24. Am Heart J. 2024. PMID: 38797460 Free PMC article.
-
Knowledge and acceptance associated with medication adherence among hypertension individuals in Aceh province, Indonesia.Heliyon. 2024 Apr 5;10(7):e29303. doi: 10.1016/j.heliyon.2024.e29303. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38617921 Free PMC article.
-
Perspective of an International Online Patient and Caregiver Community on the Burden of Spasticity and Impact of Botulinum Neurotoxin Therapy: Survey Study.JMIR Public Health Surveill. 2020 Dec 7;6(4):e17928. doi: 10.2196/17928. JMIR Public Health Surveill. 2020. PMID: 33284124 Free PMC article.
-
Patient-Reported Outcomes in ATLAS and FLAIR Participants on Long-Acting Regimens of Cabotegravir and Rilpivirine Over 48 Weeks.AIDS Behav. 2020 Dec;24(12):3533-3544. doi: 10.1007/s10461-020-02929-8. AIDS Behav. 2020. PMID: 32447500 Free PMC article.
-
"I just wanted to speak to someone- and there was no one…": using Burden of Treatment Theory to understand the impact of a novel ATMP on early recipients.Orphanet J Rare Dis. 2023 Apr 17;18(1):86. doi: 10.1186/s13023-023-02680-y. Orphanet J Rare Dis. 2023. PMID: 37069697 Free PMC article.
References
-
- Sackett DL, Haynes RB. Compliance with therapeutic regimens. 1976.
-
- Gottlieb H. Medication nonadherence: finding solutions to a costly medical problem. Drug Benefit Trends. 2000;12:57–62.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials