Hydroxychloroquine prescription trends and predictors for excess dosing per recent ophthalmology guidelines
- PMID: 29976231
- PMCID: PMC6034317
- DOI: 10.1186/s13075-018-1634-8
Hydroxychloroquine prescription trends and predictors for excess dosing per recent ophthalmology guidelines
Abstract
Background: Hydroxychloroquine (HCQ) retinopathy may be more common than previously recognized; recent ophthalmology guidelines have revised recommendations from ideal body weight (IBW)-based dosing to actual body weight (ABW)-based dosing. However, contemporary HCQ prescribing trends in the UK remain unknown.
Methods: We examined a UK general population database to investigate HCQ dosing between 2007 and 2016. We studied trends of excess HCQ dosing per ophthalmology guidelines (defined by exceeding 6.5 mg/kg of IBW and 5.0 mg/kg of ABW) and determined their independent predictors using multivariable logistic regression analyses.
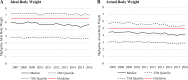
Results: Among 20,933 new HCQ users (78% female), the proportions of initial HCQ excess dosing declined from 40% to 36% using IBW and 38% to 30% using ABW, between 2007 and 2016. Among these, 47% of women were excess-dosed (multivariable OR 12.52; 95% CI 10.99-14.26) using IBW and 38% (multivariable OR 1.98; 95% CI,1.81-2.15) using ABW. Applying IBW, 37% of normal and 44% of obese patients were excess-dosed; however, applying ABW, 53% of normal and 10% of obese patients were excess-dosed (multivariable ORs = 1.61 and 0.1 (reference = normal); both p < 0.01). Long-term HCQ users showed similar excess dosing.
Conclusion: A substantial proportion of HCQ users in the UK, particularly women, may have excess HCQ dosing per the previous or recent weight-based guidelines despite a modest decline in recent years. Over half of normal-BMI individuals were excess-dosed per the latest guidelines. This implies the potential need to reduce dosing for many patients but also calls for further research to establish unifying evidence-based safe and effective dosing strategies.
Keywords: DMARDs; Epidemiology; Quality of care; Rheumatoid arthritis; Systemic lupus erythematosus.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the Partners Human Research Committee, protocol number 2017P000399. Informed consent was waived.
Competing interests
AEC, UCB; MU, UCB; AA, Exagen; MP, Anthera Inc., Glaxo Smith Kline, EMD Serono, Eli Lilly and Company, Bristol Meyer Squibb, Amgen, United Rheumatology, Global Academy, Exagen, HKC, Selecta, Horizon, AstraZeneca. The other authors have declared no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Bertsias G, Ioannidis JP, Boletis J, Bombardieri S, Cervera R, Dostal C, Font J, Gilboe IM, Houssiau F, Huizinga T, et al. EULAR recommendations for the management of systemic lupus erythematosus. Report of a task force of the EULAR standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis. 2008;67(2):195–205. doi: 10.1136/ard.2007.070367. - DOI - PubMed
-
- Alarcon GS, McGwin G, Bertoli AM, Fessler BJ, Calvo-Alen J, Bastian HM, Vila LM, Reveille JD, Group LS Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L) Ann Rheum Dis. 2007;66(9):1168–1172. doi: 10.1136/ard.2006.068676. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

