Ablation of tau causes an olfactory deficit in a murine model of Parkinson's disease
- PMID: 29976255
- PMCID: PMC6032546
- DOI: 10.1186/s40478-018-0560-y
Ablation of tau causes an olfactory deficit in a murine model of Parkinson's disease
Abstract
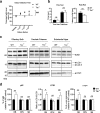
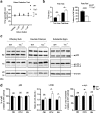
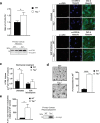
Parkinson's disease is diagnosed upon the presentation of motor symptoms, resulting from substantial degeneration of dopaminergic neurons in the midbrain. Prior to diagnosis, there is a lengthy prodromal stage in which non-motor symptoms, including olfactory deficits (hyposmia), develop. There is limited information about non-motor impairments and there is a need for directed research into these early pathogenic cellular pathways that precede extensive dopaminergic death in the midbrain. The protein tau has been identified as a genetic risk factor in the development of sporadic PD. Tau knockout mice have been reported as an age-dependent model of PD, and this study has demonstrated that they develop motor deficits at 15-months-old. We have shown that at 7-month-old tau knockout mice present with an overt hyposmic phenotype. This olfactory deficit correlates with an accumulation of α-synuclein, as well as autophagic impairment, in the olfactory bulb. This pathological feature becomes apparent in the striatum and substantia nigra of 15-month-old tau knockout mice, suggesting the potential for a spread of disease. Initial primary cell culture experiments have demonstrated that ablation of tau results in the release of α-synuclein enriched exosomes, providing a potential mechanism for disease spread. These alterations in α-synuclein level as well as a marked autophagy impairment in the tau knockout primary cells recapitulate results seen in the animal model. These data implicate a pathological role for tau in early Parkinson's disease.
Keywords: Autophagy; Neurodegeneration; Olfaction; Parkinson’s disease; Tau.
Conflict of interest statement
Ethics approval
This study was carried out in strict accordance with the Australian National Health and Medical Research Council published code of practice for animal research. The protocols were approved by The Florey Animal Ethics Committee (AEC number: 12–094 and 15–092). All efforts were made to minimize animal suffering.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
α-Synuclein BAC transgenic mice exhibit RBD-like behaviour and hyposmia: a prodromal Parkinson's disease model.Brain. 2020 Jan 1;143(1):249-265. doi: 10.1093/brain/awz380. Brain. 2020. PMID: 31816026
-
Brain infusion of α-synuclein oligomers induces motor and non-motor Parkinson's disease-like symptoms in mice.Behav Brain Res. 2017 Aug 30;333:150-160. doi: 10.1016/j.bbr.2017.06.047. Epub 2017 Jun 29. Behav Brain Res. 2017. PMID: 28668282
-
Spread of aggregates after olfactory bulb injection of α-synuclein fibrils is associated with early neuronal loss and is reduced long term.Acta Neuropathol. 2018 Jan;135(1):65-83. doi: 10.1007/s00401-017-1792-9. Epub 2017 Dec 5. Acta Neuropathol. 2018. PMID: 29209768 Free PMC article.
-
α-Synuclein in the olfactory system in Parkinson's disease: role of neural connections on spreading pathology.Brain Struct Funct. 2014 Sep;219(5):1513-26. doi: 10.1007/s00429-013-0651-2. Epub 2013 Oct 18. Brain Struct Funct. 2014. PMID: 24135772 Review.
-
Olfaction in Parkinson's disease and related disorders.Neurobiol Dis. 2012 Jun;46(3):527-52. doi: 10.1016/j.nbd.2011.10.026. Epub 2011 Dec 20. Neurobiol Dis. 2012. PMID: 22192366 Free PMC article. Review.
Cited by
-
Animal models of Alzheimer's disease: Current strategies and new directions.Zool Res. 2024 Nov 18;45(6):1385-1407. doi: 10.24272/j.issn.2095-8137.2024.274. Zool Res. 2024. PMID: 39572020 Free PMC article. Review.
-
Retinal hyperspectral imaging in mouse models of Parkinson's disease and healthy aging.Sci Rep. 2024 Jul 12;14(1):16089. doi: 10.1038/s41598-024-66284-7. Sci Rep. 2024. PMID: 38997314 Free PMC article.
-
Much More Than a Cytoskeletal Protein: Physiological and Pathological Functions of the Non-microtubule Binding Region of Tau.Front Neurol. 2020 Oct 19;11:590059. doi: 10.3389/fneur.2020.590059. eCollection 2020. Front Neurol. 2020. PMID: 33193056 Free PMC article. Review.
-
Imaging advances to detect non-motor prodromal markers of Parkinson's disease and explore therapeutic translation opportunities.NPJ Parkinsons Dis. 2025 Jun 18;11(1):174. doi: 10.1038/s41531-025-01004-0. NPJ Parkinsons Dis. 2025. PMID: 40533452 Free PMC article. Review.
-
How Well Do Rodent Models of Parkinson's Disease Recapitulate Early Non-Motor Phenotypes? A Systematic Review.Biomedicines. 2022 Nov 24;10(12):3026. doi: 10.3390/biomedicines10123026. Biomedicines. 2022. PMID: 36551782 Free PMC article. Review.
References
-
- Attems J, Jellinger KA. Olfactory tau pathology in Alzheimer disease and mild cognitive impairment. Clin Neuropathol. 2006;25:265–271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

