Flavonoids Luteolin and Quercetin Inhibit RPS19 and contributes to metastasis of cancer cells through c-Myc reduction
- PMID: 29976410
- PMCID: PMC9303038
- DOI: 10.1016/j.jfda.2018.01.012
Flavonoids Luteolin and Quercetin Inhibit RPS19 and contributes to metastasis of cancer cells through c-Myc reduction
Abstract
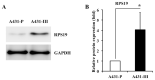
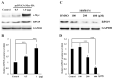
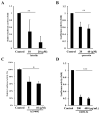
Flavonoids luteolin and quercetin can inhibit growth and metastasis of cancer cells. In our previous report, luteolin and quercetin was shown to block Akt/mTOR/c-Myc signaling. Here, we found luteolin and quercetin reduced protein level and transactivation activity of RPS19 in A431-III cells, which is isolated from parental A431 (A431-P) cell line. Further investigation the inhibitory mechanism of luteolin and quercetin on RPS19, we found c-Myc binding sites on RPS19 promoter. The Akt inhibitor LY294002, mTOR inhibitor rapamycin and c-Myc inhibitor 10058-F4 significantly suppressed RPS19 expression and transactivation activities. Overexpression and knockdown of c-Myc in cancer cells show RPS19 expression was regulated by c-Myc. Furthermore, Knockdown and overexpression of RPS19 was used to analyze of the function of RPS19 in cancer cells. The epithelial-mesenchymal transition (EMT) markers and metastasis abilities of cancer cells were also regulated by RPS19. These data suggest that luteolin and quercetin might inhibit metastasis of cancer cells by blocking Akt/mTOR/c-Myc signaling pathway to suppress RPS19-activated EMT signaling.
Keywords: EMT; Luteolin; Quercetin; RPS19; c-Myc.
Copyright © 2018. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures




Similar articles
-
Dietary flavonoids, luteolin and quercetin, inhibit invasion of cervical cancer by reduction of UBE2S through epithelial-mesenchymal transition signaling.Food Funct. 2017 Apr 19;8(4):1558-1568. doi: 10.1039/c6fo00551a. Food Funct. 2017. PMID: 28277581
-
Effects of dietary flavonoids, luteolin, and quercetin on the reversal of epithelial-mesenchymal transition in A431 epidermal cancer cells.Cancer Sci. 2011 Oct;102(10):1829-39. doi: 10.1111/j.1349-7006.2011.02035.x. Epub 2011 Aug 10. Cancer Sci. 2011. PMID: 21752154
-
Luteolin suppresses androgen receptor-positive triple-negative breast cancer cell proliferation and metastasis by epigenetic regulation of MMP9 expression via the AKT/mTOR signaling pathway.Phytomedicine. 2021 Jan;81:153437. doi: 10.1016/j.phymed.2020.153437. Epub 2020 Dec 9. Phytomedicine. 2021. PMID: 33352494
-
Inhibition of mTOR signaling by quercetin in cancer treatment and prevention.Anticancer Agents Med Chem. 2013 Sep;13(7):1025-31. doi: 10.2174/18715206113139990114. Anticancer Agents Med Chem. 2013. PMID: 23272907 Review.
-
Harnessing impaired energy metabolism in cancer cell: small molecule- mediated ways to regulate tumorigenesis.Anticancer Agents Med Chem. 2011 Mar;11(3):272-9. doi: 10.2174/187152011795347531. Anticancer Agents Med Chem. 2011. PMID: 21434854 Review.
Cited by
-
Luteolin-7-O-Glucoside Inhibits Oral Cancer Cell Migration and Invasion by Regulating Matrix Metalloproteinase-2 Expression and Extracellular Signal-Regulated Kinase Pathway.Biomolecules. 2020 Mar 26;10(4):502. doi: 10.3390/biom10040502. Biomolecules. 2020. PMID: 32224968 Free PMC article.
-
Anti-cancer properties of quercetin in osteosarcoma.Cancer Cell Int. 2021 Jul 5;21(1):349. doi: 10.1186/s12935-021-02067-8. Cancer Cell Int. 2021. PMID: 34225730 Free PMC article. Review.
-
Dietary Flavonoids Luteolin and Quercetin Inhibit Migration and Invasion of Squamous Carcinoma through Reduction of Src/Stat3/S100A7 Signaling.Antioxidants (Basel). 2019 Nov 15;8(11):557. doi: 10.3390/antiox8110557. Antioxidants (Basel). 2019. PMID: 31731716 Free PMC article.
-
Daptomycin Inhibits Multiple Myeloma Progression through Downregulating the Expression of RPS19.Comb Chem High Throughput Screen. 2025;28(4):647-653. doi: 10.2174/0113862073283460240129104114. Comb Chem High Throughput Screen. 2025. PMID: 38454765
-
Luteolin and cancer metastasis suppression: focus on the role of epithelial to mesenchymal transition.Med Oncol. 2021 May 5;38(6):66. doi: 10.1007/s12032-021-01508-8. Med Oncol. 2021. PMID: 33950369 Review.
References
-
- Kao WT, Lin CY, Lee LT, Lee PP, Hung CC, Lin YS, et al. Investigation of MMP-2 and -9 in a highly invasive A431 tumor cell sub-line selected from a Boyden chamber assay. Anticancer Res. 2008;28:2109–20. - PubMed
-
- Lin CY, Tsai PH, Kandaswami CC, Lee PP, Huang CJ, Hwang JJ, et al. Matrix metalloproteinase-9 cooperates with transcription factor Snail to induce epithelial-mesenchymal transition. Cancer Sci. 2011;102:815–27. - PubMed
-
- Lin YS, Tsai PH, Kandaswami CC, Cheng CH, Ke FC, Lee PP, et al. Effects of dietary flavonoids, luteolin, and quercetin on the reversal of epithelial-mesenchymal transition in A431 epidermal cancer cells. Cancer Sci. 2011;102:1829–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
