Virological and Immunological Outcomes of Coinfections
- PMID: 29976554
- PMCID: PMC6148187
- DOI: 10.1128/CMR.00111-17
Virological and Immunological Outcomes of Coinfections
Abstract
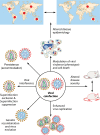
Coinfections involving viruses are being recognized to influence the disease pattern that occurs relative to that with single infection. Classically, we usually think of a clinical syndrome as the consequence of infection by a single virus that is isolated from clinical specimens. However, this biased laboratory approach omits detection of additional agents that could be contributing to the clinical outcome, including novel agents not usually considered pathogens. The presence of an additional agent may also interfere with the targeted isolation of a known virus. Viral interference, a phenomenon where one virus competitively suppresses replication of other coinfecting viruses, is the most common outcome of viral coinfections. In addition, coinfections can modulate virus virulence and cell death, thereby altering disease severity and epidemiology. Immunity to primary virus infection can also modulate immune responses to subsequent secondary infections. In this review, various virological mechanisms that determine viral persistence/exclusion during coinfections are discussed, and insights into the isolation/detection of multiple viruses are provided. We also discuss features of heterologous infections that impact the pattern of immune responsiveness that develops.
Keywords: attrition; bystander protection; coinfection; cross reactivity; diverse TCR repertoire; exclusion; persistence; virus.
Copyright © 2018 American Society for Microbiology.
Figures






References
-
- Moschidou P, Martella V, Lorusso E, Desario C, Pinto P, Losurdo M, Catella C, Parisi A, Banyai K, Buonavoglia C. 2011. Mixed infection by Feline astrovirus and Feline panleukopenia virus in a domestic cat with gastroenteritis and panleukopenia. J Vet Diagn Invest 23:581–584. doi: 10.1177/1040638711404149. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

