CD4 T Cells Reactive to Hybrid Insulin Peptides Are Indicators of Disease Activity in the NOD Mouse
- PMID: 29976617
- PMCID: PMC6110316
- DOI: 10.2337/db18-0200
CD4 T Cells Reactive to Hybrid Insulin Peptides Are Indicators of Disease Activity in the NOD Mouse
Abstract
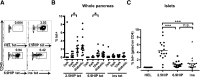
We recently established that hybrid insulin peptides (HIPs), formed in islet β-cells by fusion of insulin C-peptide fragments to peptides of chromogranin A or islet amyloid polypeptide, are ligands for diabetogenic CD4 T-cell clones. The goal of this study was to investigate whether HIP-reactive T cells were indicative of ongoing autoimmunity. MHC class II tetramers were used to investigate the presence, phenotype, and function of HIP-reactive and insulin-reactive T cells in NOD mice. Insulin-reactive T cells encounter their antigen early in disease, but they express FoxP3 and therefore may contribute to immune regulation. In contrast, HIP-reactive T cells are proinflammatory and highly diabetogenic in an adoptive transfer model. Because the frequency of antigen-experienced HIP-reactive T cells increases over progression of disease, they may serve as biomarkers of autoimmune diabetes.
© 2018 by the American Diabetes Association.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

